阅读短文,选择正确答案。
"Who has more questions, a teacher or a student?" About this question a great learned man told his student
that nobody does but a teacher.
The student got puzzled. With a smile, the teacher drew two circles (圆). Within (在……里面) the larger
one is my knowledge of things, and within the smaller one is yours. Out of the circles is still unknown to both
of us. Since mine is larger, as you can see, the line that marks out the circle is longer. That makes it clear that
who has more chances (机会) to face something still unknown.
1. The great learned man believed that ________. [ ]
A. a teacher has more questions
B. a student has more questions
C. both a teacher and a student have questions
D. everyone, except a teacher, has more questions
2. The student thought that ________. [ ]
A. a student's knowledge is less than his teacher's because it comes from the teacher
B. a teacher can not necessarily answer all the questions his students ask
C. those who have less knowledge have more questions
D. anyone who learns more has more questions
3. At the end of the story, the great learned man concluded (推断) that ________. [ ]
A. a student should learn from his teacher
B. a teacher does not have so many questions as his student
C. a student knows more than his teacher
D. a teacher has more chances to face what he doesn't know
4. Which of the following topics can NOT express the idea of the story? [ ]
A. You Will Never Learn Enough.
B. A Teacher and His Student.
C. There Is No Limit to Knowledge.
D. One Is Never Too Old to Learn.
5. We can often find such an article in ________. [ ]
A. the Palace Museum
B. any book
C. a newspaper of magazine
D. An encyclopedia (百科全书)
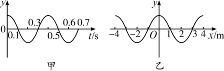
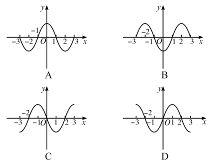
 ,可见,在t′时刻的波形相对于图乙有两种可能:①如波沿x轴正方向传播,则应为D图;②如波沿x轴负方向传播,则应为C图.
,可见,在t′时刻的波形相对于图乙有两种可能:①如波沿x轴正方向传播,则应为D图;②如波沿x轴负方向传播,则应为C图.

 ,可见,在t′时刻的波形相对于图乙有两种可能:①如波沿x轴正方向传播,则应为D图;②如波沿x轴负方向传播,则应为C图.
,可见,在t′时刻的波形相对于图乙有两种可能:①如波沿x轴正方向传播,则应为D图;②如波沿x轴负方向传播,则应为C图.