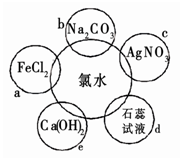
氯水具有多种性质,根据新制氯水分别与如图五种物质发生的反应填空(氯水足量):
(1)a、b、c中反应的离子方程式为: 。
e中的化学反应方程式为 。上述反应中发生了氧化还原反应的是: (填“a”、“b”、“c”或“e”)。
(2)能证明氯水具有漂白性的现象是____________________________。
(3)久置的氯水变为________,用化学反应方程式表示为__________。
(4)实验室保存饱和氯水的方法是_____________________________。
(1)2Fe2++Cl2===2Fe3++2Cl-(1分)
2H++CO32-===H2O+CO2↑(1分)
Ag++Cl-===AgCl↓(1分)
2Cl2+2Ca(OH)2===Ca(ClO)2+CaCl2+2H2O ,(2分)
ae(1分)
(2)氯水与石蕊试液反应,先变红后褪色(1分)
(3)稀盐酸(1分) 2HClO 2HCl+O2↑(1分)
2HCl+O2↑(1分)
(4)在阴凉处置于棕色试剂瓶中密封保存(1分)
题目分析:(1)a为Cl2氧化Fe2+,离子方程式:Cl2+2Fe2+=2Cl‾+2Fe3+,b为氯水中的盐酸与Na2CO3,离子方程式为:CO32‾+2H+= H2O+CO2↑,c为氯水中的Cl‾与AgNO3溶液中的Ag+反应,离子方程式为:Cl‾+Ag+===AgCl↓,e为Cl2与Ca(OH)2反应,离子方程式为:2Cl2+2Ca(OH)2===Ca(ClO)2+CaCl2+2H2O ,ae中反应元素化合价发生了变化,为氧化还原反应。
(2)把新制氯水加入石蕊试液,氯水显酸性使石蕊变红,HClO使石蕊褪色,说明氯水具有漂白性。
(3)HClO分解生成HCl和O2,所以久置的氯水变为稀盐酸,化学方程式为:2HClO 2HCl+O2↑
2HCl+O2↑
(4)因为HClO在光照条件下易分解,所以氯水在阴凉处置于棕色试剂瓶中密封保存。
