切开的金属Na暴露在空气中,其变化过程如下:

(1)反应Ⅰ的反应过程与能量变化的关系如下:
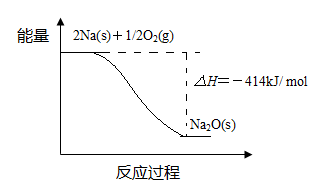
① 反应Ⅰ 是 反应(填“放热”或“吸热”),判断依据是 。
② 1 mol Na(s)全部氧化成Na2O(s)的热化学方程式是 。
(2)反应Ⅱ是Na2O与水的反应,其产物的电子式是 。
(3)白色粉末为Na2CO3。将其溶于水配制为0.1 mol/L Na2CO3溶液,下列说法正确的是 (填字母)。
A.升高温度,溶液的pH降低
B.c(OH-)-c (H+)=c (HCO3-)+2 c (H2CO3)
C.加入少量NaOH固体,c (CO32―)与c (Na+)均增大
D.c (Na+) > c (CO32―) > c (HCO3―) > c(OH―) > c (H+)(4) 钠电池的研究开发在一定程度上可缓和因锂资源短缺引发的电池发展受限问题。
① 钠比锂活泼,用原子结构解释原因_______。
②ZEBRA 电池是一种钠电池,总反应为NiCl2 + 2Na  Ni + 2NaCl。其正极反应式是_____。
Ni + 2NaCl。其正极反应式是_____。
(1)①放热 反应物总能量高于生成物总能量
② Na(s)+1/4O2(g)=1/2Na2O(s) △H=-207 kJ/ mol
(2) (3)B C
(3)B C
(4)① 最外层电子数相同,随着核电荷数增加,原子半径逐渐增大,金属性增强。
② NiCl2+ 2Na++ 2e-=Ni +2NaCl
题目分析:(1)①由于反应物的总能量比生成物的总能量高,所以发生的反应Ⅰ是放热反应。② 由题目提供的能量关系可得该反应的热化学方程式为:Na(s)+1/4O2(g)=1/2Na2O(s) △H=-207 kJ/ mol。(2)反应Ⅱ是Na2O与水的反应,Na2O与水反应产生NaOH,反应的方程式为Na2O+H2O =2NaOH。NaOH的电子式为: 。(3)A.Na2CO3是强碱弱酸盐,在溶液中发生水解反应:Na2CO3+H2O
。(3)A.Na2CO3是强碱弱酸盐,在溶液中发生水解反应:Na2CO3+H2O NaHCO3+ NaOH。盐的水解反应是吸热反应,所以升高温度,促进盐的水解,溶液的碱性增强,溶液的pH增大。错误。B.根据电荷守恒可得c (H+)+ c (Na+)= c(OH-)+ c (HCO3-)+ 2c (CO32―);根据物料守恒可得c (Na+)=" 2" c (H2CO3)+ 2c (HCO3-)+ 2c (CO32―),两式相减,整理可得c(OH-)-c (H+)=c (HCO3-)+2 c (H2CO3)。正确。C.加入少量NaOH固体,由于Na+物质的量增多。c (Na+)增大,c(OH-)增大,使平衡Na2CO3+H2O
NaHCO3+ NaOH。盐的水解反应是吸热反应,所以升高温度,促进盐的水解,溶液的碱性增强,溶液的pH增大。错误。B.根据电荷守恒可得c (H+)+ c (Na+)= c(OH-)+ c (HCO3-)+ 2c (CO32―);根据物料守恒可得c (Na+)=" 2" c (H2CO3)+ 2c (HCO3-)+ 2c (CO32―),两式相减,整理可得c(OH-)-c (H+)=c (HCO3-)+2 c (H2CO3)。正确。C.加入少量NaOH固体,由于Na+物质的量增多。c (Na+)增大,c(OH-)增大,使平衡Na2CO3+H2O NaHCO3+ NaOH逆向移动,所以c (CO32―)也增大。正确。D.Na2CO3=2Na++ CO32―;c (Na+) > c (CO32―);H2O+CO32-
NaHCO3+ NaOH逆向移动,所以c (CO32―)也增大。正确。D.Na2CO3=2Na++ CO32―;c (Na+) > c (CO32―);H2O+CO32- OH-+HCO3-,所以c(OH―) > c (H+)。由于在溶液中还存在H2O
OH-+HCO3-,所以c(OH―) > c (H+)。由于在溶液中还存在H2O H++ OH―。所以c c(OH―) > (HCO3―) 。因此在溶液微粒的浓度关系为c (Na+) > c (CO32―) > c(OH―) > c (HCO3―) > c (H+)。错误。(4)①Na、Li都是第一主族的元素,由于原子半径Na>Li。原子半径越大,原子失去电子的能力就越强,因此钠比锂活泼。②由总方程式可知该电池的正极电极式为NiCl2+ 2Na++ 2e-=Ni +2NaCl;负极的电极式为2Na-2e-=2Na+.
H++ OH―。所以c c(OH―) > (HCO3―) 。因此在溶液微粒的浓度关系为c (Na+) > c (CO32―) > c(OH―) > c (HCO3―) > c (H+)。错误。(4)①Na、Li都是第一主族的元素,由于原子半径Na>Li。原子半径越大,原子失去电子的能力就越强,因此钠比锂活泼。②由总方程式可知该电池的正极电极式为NiCl2+ 2Na++ 2e-=Ni +2NaCl;负极的电极式为2Na-2e-=2Na+.
