(本题16分)钼酸钠晶体(Na2MoO4·2H2O)是无公害型冷却水系统的金属缓蚀剂,由钼精矿(主要成分是MoS2,含少量PbS等)制备钼酸钠晶体的部分流程如下:
 (1)焙烧的过程中采用的是“逆流焙烧”的措施,则该措施的优点是:①_______________
(1)焙烧的过程中采用的是“逆流焙烧”的措施,则该措施的优点是:①_______________
②____________________________
(2)写出焙烧时生成MoO3的化学方程式为:______________________________________
(3)写出“碱浸”反应的离子方程式:
(4)重结晶得到的母液可以在下次重结晶时重复使用,但达到一定次数后必须净化处理,原因是 。
(5)下图是碳钢在3种不同介质中的腐蚀速率实验结果:
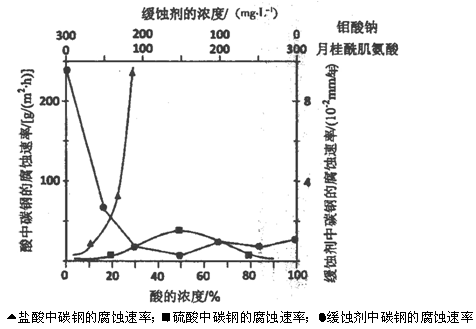
①碳钢在盐酸和硫酸中腐蚀速率随酸的浓度变化有明显差异,其原因可能是 ________________。
②空气中钼酸盐对碳钢的缓蚀原理是在钢铁表面形成FeMoO4—Fe2O3保护膜。
密闭式循环冷却水系统中的碳钢管道缓蚀,除需加入钼酸盐外还需加入NaNO2。则NaNO2的作用是 _________________________________________。
③若缓释剂钼酸钠—月桂酸肌氨酸总浓度为300mg·L-1,则缓蚀效果最好时钼酸钠的物质的量浓度为
(6)锂和二硫化钼形成的二次电池的总反应为:xLi + nMoS2 Lix(MoS2)n。则电池放电时的正极反应式是:___________________________________。
Lix(MoS2)n。则电池放电时的正极反应式是:___________________________________。
(1)①增长钼精矿与空气接触的时间,使其充分反应,提高原料的利用率 (1分)
②实现热量交换,节约能源 (1分)
(2)2MoS2 + 7O2 = 2MoO3 +4 SO2(高温)(2分)
(3)MoO3+CO32-= MoO42-+CO2↑ (2分)
(4)多次使用后母液中杂质的浓度增大,再次重结晶时会析出杂质影响产品纯度(2分)
(5)①Cl-有利于碳钢的腐蚀,SO42-不利于碳钢的腐蚀,使得钢铁在盐酸中的腐蚀速率明显快于硫酸;(1分)
硫酸溶液随着浓度的增大,氧比性增强,会使钢铁钝化,腐蚀速率减慢 (1分)
②替代空气中氧气起氧化剂作用 (2分)
③7.28×l0-4mol·L-1(2分)
(6)nMoS2 + xLi+ + xe- = Lix(MoS2)n (2分)
题目分析:要弄懂原料及制备的产品,流程的第一步尽可能看懂。
(1)审题“逆流焙烧”在课本找到类似的是冷凝管中水流与气流的方向是相对,为的是冷却效果更好,由此可知,“逆流焙烧”当然也为了增加接触时间,提高原料利率;接触时间长,热量交换也更充分,能节约能源。
(2)先写出MoS2 + O2 = MoO3 + 这一部分,可推断出另一产物为二氧化硫,利用氧化还原反应得失电子守恒和原子守恒配平。
(3)首先找到“碱浸”在流程图中的位置,注意,箭头指入的是加入的试剂,而指出的是产物,即加入Na2CO3,出来了CO2,还有MoO42-应从最后产物中获取信息,即MoO3+CO32-= MoO42-+CO2↑
(4)重结晶的目的是提纯产品,母液当然含有一定量的产品,也含有一定量的杂质,所以到一定程度时,要进行净化,因为里面的杂质含量高了。
(5)①此题一定要看懂图中横坐标与纵坐标所表示的是什么量,由图中可知,相同的酸度下,腐蚀速率不一样,可知是因为Cl-与SO42-的不同导致的;再有是Fe在浓硫酸或浓硝酸中会钝化,所以,当酸度增大时,Fe表面发生钝化也会使腐蚀速率下降。
②亚硝酸钠的作用,应从其性质去想,一是氧化性,二是强碱弱酸盐,在这里结合题目应用到其氧化性。
③由坐标图中可读出当钼酸钠—月桂酸肌氨酸浓度为150 mg/L、150mg/L时,腐蚀速率是最低的,钼酸钠的浓度由质量浓度换算成物质的量浓度即可,(150×10-3g÷206g/mol)÷1L=7.28×l0-4mol·L-1
(6)放电时,正极应是发生还原反应的,从总反应式中找到发生还原反应的物质为MoS2,而被还原的产物为Lix(MoS2)n,即nMoS2+ e- + =Lix(MoS2)n,接下来是确定电子数和Li+的个数,根据电荷守恒,电子数与Li+的个数是相等的,电极反应式也就完成了。
