短文改错(共10小题;每小题1分,满分10分)
假定英语课上老师要求同桌之间交换修改作文,请你修改你同学写的以下作文。文中共有10处语言错误,每句中最多有两处。错误涉及一个单词的增加、删除或修改。
增加:在缺词处加一个漏字符号(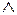 ),并在其下面写出该加的词。
),并在其下面写出该加的词。
删除:把多余的词用斜线(\)划掉。
修改:在错的词下划一横线,并在该词下面写出修改后的词。
注意:1. 每处错误及其修改均仅限一词;
2.只允许修改10处,多者(从第11处起)不计分。
Dear Mrs. Winthorp,
So my first week at college is over! No lectures this morning so I think I’d email you and let you know what things are going.
I’m glad to say that anything has worked out fine in the dorm. I remember asking for a room as far away from the lifts as possible and they find me a comfortable one on second floor. There are two girls from my course here and I plan to make friend with them so they we can help each other on the course work. Everyone else seems very nice and warmly here.
Well, I had better to stop now. I’m going to attend mine first lesson this afternoon, for I’ve got some preparation to make, Keep in touch.
Best,
Carol
Dear Mrs. Winthrop,
So my first week at college is over! No lectures this morning so I think I’d email you and let you know what things are going.
how
I’m glad to say that anything has worked out fine in the dorm. I remember asking for a room as
everything
far away from the lifts as possible and they find me a comfortable one on 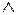 second floor. There
second floor. There
the
are two girls from my course here and I plan to make friend with them so they we can help each
friends
other on the course work. Everyone else seems very nice and warmly here.
with warm
Well, I had better to stop now. I’m going to attend mine first lesson this afternoon, for I’ve got
my so
some preparation to make, Keep in touch.
Best,
Carol
