(16分)肼(N2H4)又称联氨,是一种可燃性液体,与氧气或氮氧化物反应均可生成氮气和水。氢气是一种清洁能源,液氢和肼均可用作火箭燃料。
Ⅰ氢气的制取与储存是氢能源利用领域的研究热点。
已知: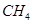 (g)+
(g)+ 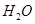 (g)=
(g)=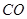 (g)+
(g)+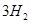 (g)
(g) 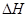 = +
= +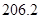
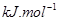
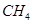 (g)+
(g)+  (g)=
(g)=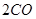 (g)+
(g)+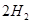 (g)
(g)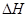 = +
= +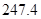
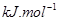
(1)氢气作为新能源的优点 。(答2点)
(2)以甲烷为原料制取氢气是工业上常用的制氢方法。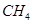 (g)与
(g)与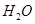 (g)反应生成
(g)反应生成 (g)和
(g)和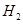 (g)的热化学方程式为 。
(g)的热化学方程式为 。
(3)H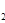 O的热分解也可得到H
O的热分解也可得到H ,高温下水分解体系中主要气体的体积分数与温度的关系如图所示。图中A、B表示的物质依次是 、 。
,高温下水分解体系中主要气体的体积分数与温度的关系如图所示。图中A、B表示的物质依次是 、 。
Ⅱ(4)肼一空气燃料电池是一种碱性燃料电池,电解质溶液是20%—30%的KOH溶液。该电池放电时,负极的电极反应式是 。
(5)下图是一个电化学装置示意图。用肼一空气燃料电池做此装置的电源。
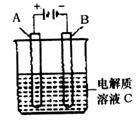
①如果A是铂电极,B是石墨电极,C是硫酸—硫酸铵,阴极的电极反应式是 。
②利用该装置可制得少量过氧化氢:在阳极上SO42—被氧化成S2O82—(过二硫酸根离子),S2O82—与H2O反应生成H2O2,S2O82—+2H2O=2SO42—+H2O2+2H+。若要制取2molH2O2,该燃料电池理论上需消耗 molN2H4。
(6)由A、B、C、D四种金属按下表中装置进行实验。
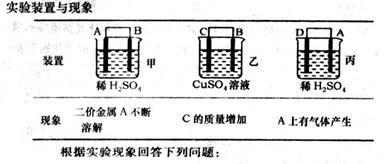
①装置丙中溶液的P H 。(填“变大”“变小”或“不变”)
②四种金属活泼性由弱到强的顺序是 。
(1)无污染, 资源丰富、热值高等 ( 各1分)
(2) CH4(g)+2H2O(g)=CO2(g)+4H2(g) ΔH=+165KJ.mol-1
(3)氢原子、氧原子(各1分)(4) N2H4-4e-+4OH-=N2+4H2O
(5)① 2H++2e-=H2 ↑② 1 (6)① 变大 ② CBAD (每空2分)
(1)考查氢气作为能源的特点。根据氢气的燃烧热和氢气的来源可知,氢气作为新能源的优点生成物是水没有污染,资源丰富,热值高等。
(2)考查盖斯定律的应用,根据反应①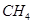 (g)+
(g)+ 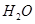 (g)=
(g)=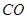 (g)+
(g)+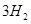 (g)和反应②
(g)和反应②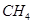 (g)+
(g)+ 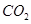 (g)=
(g)=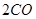 (g)+
(g)+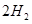 (g)可知,①×2-②即得到CH4(g)+2H2O(g)=CO2(g)+4H2(g),所以反应的反应热是+
(g)可知,①×2-②即得到CH4(g)+2H2O(g)=CO2(g)+4H2(g),所以反应的反应热是+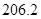
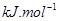 ×2-
×2-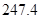
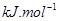 =+165KJ.mol-1,即热化学方程式为
=+165KJ.mol-1,即热化学方程式为
CH4(g)+2H2O(g)=CO2(g)+4H2(g) ΔH=+165KJ.mol-1。
(3)水分解的化学方程式为2H2O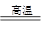 2H2+O2,即氢气的和氧气的体积之比是2︰1,所以根据图像可判断,A表示的是氢原子,B表示的是氧原子。
2H2+O2,即氢气的和氧气的体积之比是2︰1,所以根据图像可判断,A表示的是氢原子,B表示的是氧原子。
(4)原电池中负极是失去电子的,所以肼在负极发生氧化反应,电解质是碱性溶液,所以电极反应式为N2H4-4e-+4OH-=N2+4H2O。
(5)电解池中阴极是得到电子的,发生还原反应,B电极和电源的负极相连,所以B是阴极,溶液中中氢离子放电,电极反应式为2H++2e-=H2↑;根据反应式可知要制取2molH2O2,则消耗2mol S2O82—。S2O82—中氧的化合价是-7/4价,SO42—中氧的化合价是-2价,所以生成2mol S2O82—失去的电子是(2-7/4)×8×2=4mol。1mol肼失去4mol电子,根据得失电子守恒可知,消耗肼的物质的量是1mol。
(6)丙是原电池,A极产生氢气,所以A是正极,D负极,所以丙中pH变大。甲中A不断溶解,说明A是负极,B是正极。乙中C极质量增加,所以C是正极,B是负极,所以四种金属活泼性由弱到强的顺序是CBAD
