(10分)(1) 氨是氮循环中的重要物质,氨的合成是目前普遍使用的人工固氮方法。
(1)已知:H-H键能为436KJ·mol-1,N≡N键能为945 KJ·mol-1,N-H键能为391 KJ·mol-1。写出合成氨反应的热化学方程式:
(2)可逆反应N2 +3H2 2NH3 在恒容密闭容器中进行,达到平衡状态的标志是
2NH3 在恒容密闭容器中进行,达到平衡状态的标志是
①单位时问内生成n mo1 N2的同时生成3n mol H2
②单位时间内1个N≡N键断裂的同时,有6个N—H键断裂
③容器中N2、H2、NH3的物质的量为1:3:2
④常温下,混合气体的密度不再改变的状态
⑤常温下,混合气体的平均相对分子质量不再改变的状态
(3)恒温下,往一个2L的密闭容器中充入2.6 mol H2和1 mol N2,反应过程中对NH3的浓度进行检测,得到的数据如下表所:
| 时间/min | 5 | 10 | 15 | 20 | 25 | 30 |
| C(NH3)/mol·L-1 | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
(4)氨是氮肥工业的重要原料。某化肥厂以天然石膏矿(主要成分CaSO4)为原料生产铵态氮肥(NH4)2SO4,(已知Ksp(CaSO4)=7.10×10-5 Ksp(CaCO3)=4.96×10-9)其工艺流程如下:
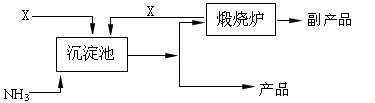
请写出制备(NH4)2SO4的反应方程式: ;
并利用有关数据简述上述反应能发生的原因
(1)N2(g) + 3H2(g)  2NH3(g);△H=-93 KJ·mol-1(2分)
2NH3(g);△H=-93 KJ·mol-1(2分)
(2)②⑤(选对1个1分,错选扣分,不出现负分,共2分)
(3)0.008 mol·L-1·min-1(1分) 0.1(1分) 逆反应(1分)
(4)CaSO4+(NH4)2CO3= (NH4)2SO4 +CaCO3↓(2分)
因为Ksp(CaSO4)=7.10×10-5>Ksp(CaCO3)=4.96×10-9(1分)
(1)反应热就是断键吸收的能量和形成化学键所放出的能量的差值,即436KJ·mol-1×3+945 KJ·mol-1-391 KJ·mol-1×6=-93 KJ·mol-1,所以热化学方程式为N2(g) + 3H2(g)  2NH3(g);△H=-93 KJ·mol-1。
2NH3(g);△H=-93 KJ·mol-1。
(2)在一定条件下,当可逆反应的正反应速率和逆反应速率相等时(但不为0),反应体系中各种物质的浓度或含量不再发生变化的状态,称为化学平衡状态。①中反应速率的方向是相同的,不正确。
②中反应速率的方向相反,且满足速率之比是相应的化学计量数之比,正确。平衡时浓度不再发生变化,但物质之间的浓度不一定相等或满足某种关系,所以③不正确。密度是混合气的质量和容器容积的比值,在反应过程中质量和容积始终是不变的,④不正确。混合气的平均相对分子质量是混合气的质量和混合气的总的物质的量的比值,质量不变,但物质的量是变化的,所以⑤可以说明。答案选②⑤。
(3)5min时氨气的浓度是0.08mol/L,所以氨气的反应速率是0.08mol/L÷5min=0.016mol/(L·min)。因为速率之比是相应的化学计量数之比,所以氮气的反应速率是
0.008mol/(L·min)。平衡时氨气的浓度是0.20mol/L,则消耗氮气和氢气的浓度分别是0.1mol/L、0.3mol/L。则平衡时氮气和氢气的浓度分别是0.5mol/L-0.1mol/L=0.4mol/L、1.3mol/L-0.3mol/L=1.0mol/L,所以平衡常数是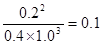 。若往平衡体系中加入H2、N2和NH3各2mol,则此时H2、N2和NH3的浓度分别为2.0mol/L、1.4mol/L、1.2mol/L,所以
。若往平衡体系中加入H2、N2和NH3各2mol,则此时H2、N2和NH3的浓度分别为2.0mol/L、1.4mol/L、1.2mol/L,所以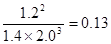 >0.1,所以平衡向逆反应方向移动。
>0.1,所以平衡向逆反应方向移动。
(4)碳酸钙比硫酸钙的溶解度小,即Ksp(CaSO4)=7.10×10-5>Ksp(CaCO3)=4.96×10-9,所以反应的方程式为CaSO4+(NH4)2CO3= (NH4)2SO4 +CaCO3↓。
