某无色溶液,其中有可能存在的离子如下:Na+、Ag+、Ba2+、Al3+、AlO2-、S2-、CO32-、SO32- 、SO42-。现取该溶液进行有关实验,实验结果如下图所示:
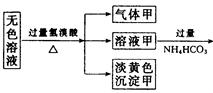
(1)沉淀甲是 ,生成沉淀的离子方程式 。
(2)沉淀乙是 ,由溶液甲生成沉淀乙的离子方程式 。
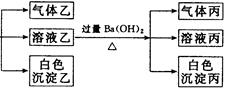
(3)沉淀丙是 。
(4)综合上述信息,可以肯定存在的离子有 。
(1)S; 2S2-+SO32-+6H+=3S↓+3H2O
(2)Al(OH)3; Al3++3HCO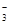 =Al(OH)3↓+3CO2↑
=Al(OH)3↓+3CO2↑
(3)BaCO3, 可能有BaSO4; 在沉淀丙中加入足量盐酸,若沉淀不能全部溶解,说明除BaCO3外还有BaSO4;若全部溶解,则仅有BaCO3.
(4) S2-、SO 、AlO
、AlO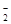 、Na+
、Na+
本题的突破口是沉淀甲、乙、丙的成分,加入过量的氢溴酸生成淡黄色沉淀甲,回顾中学化学中常见的淡黄色物质有AgBr、S、Ag3PO4等。这里应该排除排除Ag3PO4的,关键甲是AgBr还是S?若是AgBr沉淀的话,由于Ag+与题干中的4种阴离子均不能大量共存,从而否决了Ag+。可知甲是S,显然此处的S是溶液中S2-和SO32-在酸性条件下发生的归中反应生成的。由此推出原溶液中存在S2-和SO32-。由于有SO32-,所以一定不存在Ba2+、Fe3+。根据电中性原则,溶液中必须有阳离子,故一定存在Na+。气体甲可能是HBr与过量的S2-反应生成的H2S,也可能是HBr与过量的SO32-反应生成的SO2,也可能是HBr与原溶液中可能存在的CO32-反应生成的CO2。过量的NH4HCO3中和多余的H+后,又反应生成白色沉淀乙,对照题干中的离子(Ag+、Ba2+、Fe3+都不存在),推出沉淀乙只可能是Al元素引起的Al(OH)3沉淀,即HCO3-与Al3+发生双水解所造成的,而Al3+来自于AlO2-与过量的氢溴酸反应生成的,所以原溶液中一定存在AlO2-。气体乙是HCO3-与剩余的H+以及Al3+反应产生的,只能是CO2。Ba(OH)2与上步过量的NH4HCO3反应,生成白色沉淀丙,即BaCO3沉淀,另外,也可能存在BaSO4沉淀,但无法确定原溶液中是否含有CO32-和SO42-。气体丙一定是NH4+与过量的Ba(OH)2反应生成的NH3。
综上所述,肯定存在的离子有:Na+、AlO2-、S2-、SO32-;一定不存在的离子有:Ag+、Ba2+、Fe3+;可能存在的离子有:CO32-、SO42-。
