(1)写出下列物质发生水解反应的离子方程式,并指出水溶液的酸碱性:
①NaHS_________________
②NaHPO4_________________
③CH3COONH4_________________
④NaHSO3_________________
⑤NaH2PO4_________________
⑥CuSO4_________________
(2)写出下列各组盐溶液混合后发生反应的离子方程式:
①FeCl3和Na2CO3_________________
②CuSO4和Na2S_________________
③AlCO3和Na2S_________________
④Na2SiO3和NH4Cl_________________
⑤Al2(SO4)3和Na2CO3_________________
⑥Al2(SO4)3和NaHCO3_________________
(1)①HS-+H2O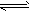 H2S+OH-(碱性);②HPO4-+H2O
H2S+OH-(碱性);②HPO4-+H2O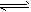 H2PO4-+OH-(碱性)、HPO42-+H2O
H2PO4-+OH-(碱性)、HPO42-+H2O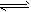
H2PO4+OH-(碱性);③CH3COO-+NH4++H2O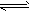 CH3COOH+NH3·H2O(中性);④HSO3-+H2O
CH3COOH+NH3·H2O(中性);④HSO3-+H2O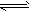
H2SO3+OH-(酸性);⑤H2PO4-+H2O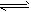 H3PO4+OH-(酸性);⑥Cu2++2H2O
H3PO4+OH-(酸性);⑥Cu2++2H2O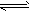 Cu(OH)2+2H+(酸性)
Cu(OH)2+2H+(酸性)
(2)①2Fe3++3CO32-+3H2O=2Fe(OH)3↓+3CO2↑;②Cu2++S2-=CuS↓;③2Al3++3S2-+6H2O=2Al(OH)3↓+
3H2S↑;④2NH4++SiO32-=2NH3↑+H2SiO3↓;⑤2Al3++3CO32-+3H2O=2Al(OH)3↓+3CO2↑
⑥Al3++3HCO3-=Al(OH)3↓+3CO2↑
