(5分)将6.5g石灰石样品投入到36.5g稀盐酸溶液中制取二氧化碳气体,恰好完全反应。反应后将溶液过滤、干燥,称量剩余固体残渣的质量为1.5g(提示:①石灰石样品中的杂质不溶于水,也不发生反应;②反应后生成的氯化钙完全溶解)。计算:
(1)该石灰石样品中碳酸钙的质量是多少?
(2)反应后所得溶液的溶质质量分数为多少?(计算结果保留一位小数)
解:(1)碳酸钙的质量:6.5g-1.5g=5g………………………………(1分)
(2)设反应后生成的氯化钙质量为x,生成的二氧化碳质量为y:
CaCO3+2HCl=CaCl2+CO2↑+H2O…………………(1分)
100 111 44
5g x y
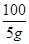 =
=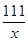 x=5.55g
x=5.55g
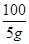 =
=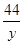 y=2.2g………………………………(1分)
y=2.2g………………………………(1分)
反应后所得溶液质量为:5g+36.5g-2.2g=39.3g
反应后所得溶液的溶质质量分数为: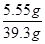 ×100%=14.1%………………(1分)
×100%=14.1%………………(1分)
答:该石灰石样品中碳酸钙的质量为5克,反应后所得的溶液的溶质质量分数为14.1%。………………………………………(设问和答共1分)
其它合理解答均可得分。
分析:(1)由样品和剩余固体的质量即可求得该石灰石样品中碳酸钙的质量;
(2)由反应的碳酸钙的质量,根据反应方程式即可求得生成氯化钙和二氧化碳的质量;再根据质量守恒定律即可求得反应后溶液的质量;最后根据溶质质量分数= ×100%即可求得反应后所得溶液的溶质质量分数.
×100%即可求得反应后所得溶液的溶质质量分数.
解:(1)碳酸钙的质量:6.5g-1.5g=5g;
(2)设反应后生成的氯化钙质量为x,生成的二氧化碳质量为y:
CaCO3+2HCl=CaCl2+CO2↑+H2O
100 111 44
5g x y
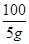 =
=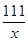 x=5.55g
x=5.55g
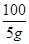 =
=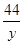 y=2.2g
y=2.2g
反应后所得溶液质量为:5g+36.5g-2.2g=39.3g;
反应后所得溶液的溶质质量分数为: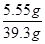 ×100%=14.1%;
×100%=14.1%;
答:该石灰石样品中碳酸钙的质量为5克,反应后所得的溶液的溶质质量分数为14.1%.
点评:求反应后所得溶液的质量的计算是初中化学计算的一个重点内容,其方法一般是:所加入的所有物质的质量总和-生成气体的质量-生成沉淀的质量.
