问题
选择题
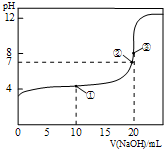
25℃时,用0.1000mol/L NaOH溶液滴定20.00mL 0.1000mol/L CH3COOH溶液所得滴定曲线如图.下列叙述正确的是( )
A.点①所示溶液:c(CH3COO-)+c(CH3COOH)=c(Na+)
B.点②所示溶液:c(Na+)=c(CH3COO-)>c(H+)=c(OH-)
C.点③所示溶液:c(Na+)>c(OH-)>c(CH3COO-)>c(H+)
D.滴定终点时:c(CH3COOH)+c(CH3COO-)=c(Na+)
答案
答案:B
