下列解释实验事实的平衡不正确的是
| 实 验 | 解释 | |
| A | 100℃ 0.1 mol/LNa2SO4溶液pH= 6.2 | H2O H+ + OH- H+ + OH- |
| B | 0.1 mol/L CH3COOH的pH=3 | CH3COOH CH3COO- + H+ CH3COO- + H+ |
| C | 配制FeCl3溶液时加少量盐酸 | Fe3+ + 3OH- Fe(OH)3 Fe(OH)3 |
| D | 用稀硫酸洗涤BaSO4,沉淀损失小 | BaSO4(s)  Ba2+(aq) + SO42-(aq) Ba2+(aq) + SO42-(aq) |
答案:C
题目分析:A、温度升高,H2O的电离平衡向右移动,正确;B、CH3COOH为弱酸,部分电离,存在电离平衡:CH3COOH 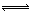 CH3COO‾+H+,正确;C、配制FeCl3溶液时加少量盐酸,目的是抑制Fe3+的水解,Fe3++3H2O
CH3COO‾+H+,正确;C、配制FeCl3溶液时加少量盐酸,目的是抑制Fe3+的水解,Fe3++3H2O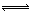 Fe(OH)3+3H+,错误;D、用稀硫酸洗涤BaSO4,使BaSO4的沉淀溶解平衡:BaSO4(s)
Fe(OH)3+3H+,错误;D、用稀硫酸洗涤BaSO4,使BaSO4的沉淀溶解平衡:BaSO4(s)  Ba2+(aq) + SO42-(aq),向左移动,正确。
Ba2+(aq) + SO42-(aq),向左移动,正确。
