已知在常温下测得浓度均为0.1mol/L的下列6种溶液的pH:则下列说法或表达正确的是
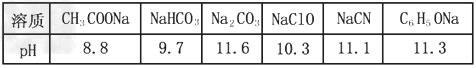
A.CO2+H2O+2NaClO==Na2CO3+2HClO
B.2HCN+Na2CO3==CO2+2NaCN+H2O
C.结合质子能力由强到弱的顺序为:CO32->HCO3->CH3COO-
D.常温下电离常数比较:Ka (H2CO3)>Ka(CH3COOH)>Ka(C6H5OH)
答案:C
已知在常温下测得浓度均为0.1mol/L的下列6种溶液的pH:则下列说法或表达正确的是

A.CO2+H2O+2NaClO==Na2CO3+2HClO
B.2HCN+Na2CO3==CO2+2NaCN+H2O
C.结合质子能力由强到弱的顺序为:CO32->HCO3->CH3COO-
D.常温下电离常数比较:Ka (H2CO3)>Ka(CH3COOH)>Ka(C6H5OH)
答案:C