书面表达。
假设你叫李华,你的美国笔友Johnson想和家人来中国定居,请你帮他在A、B两个城市中做出选择。
下面的图表是对这两座城市在就业、娱乐和环境等方面所作的对比。请你根据该表提供的信息用英语给
他写封电子邮件,提出你的建议并说明理由。
注意:1. 图表左边的数字说明人们对这两个城市的喜爱程度。
2. 词数l20左右。短文开头和结尾已为你写好,不计入总词数。
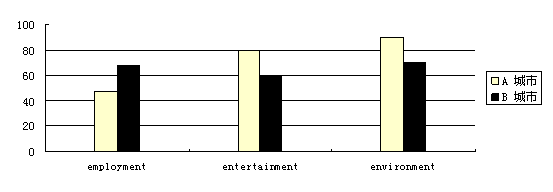
Johnson,
I'm so glad to learn that you and your family are coming to settle down in China.
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
Yours,
Li Hua
Johnson,
I'm so glad to learn that you and your family are coming to settle down in China.
After a careful study of the information about the two cities, I suggest you choose to
live in City A. First of all, City A is a more popular city to live in. The environment
in City A is better than that in City B and the climate there is very pleasant. Besides,
there are many places for entertainment where you can enjoy yourselves in your spare time.
As for the employment, though the job opportunities are not so good as in City B, yet I
don't think it's a problem for you as a teacher of English.
This is only my suggestion and it's up to you to decide. Hope to hear from you again.
Yours,
Li Hua
答案不唯一
