分别取40mL的0.50mol/L盐酸与40mL 0.55mol/L氢氧化钠溶液进行中和反应。通过测定反应过程中所放出的热量可计算中和热。请回答下列问题。
(1)理论上稀强酸、稀强碱反应生成1mol水时放出57.3kJ的热量,写出表示稀硫酸和稀氢氧化钠溶液反应的中和热的热化学方程式 ;
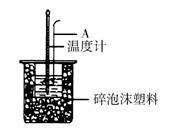
(2)如右图所示,仪器A的名称是_______________;在实验过程中,如果不把温度计上的酸用水冲洗干净直接测量NaOH溶液的温度,则测得的△H (填“偏大”、“偏小”或“无影响”);
(3)假设盐酸和NaOH溶液的密度都是1g/cm3,又知中和后生成溶液的比热容c=4.18×10-3 kJ/(g·℃)。为了计算中和热,某学生实验记录数据如下:
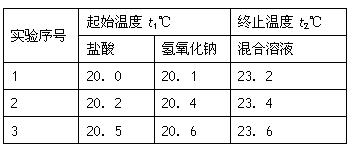
依据该学生的实验数据计算,该实验测得的中和热△H=___________;(结果保留一位小数)
(4)若用0.50mol/L醋酸代替盐酸和氢氧化钠溶液反应则测得中和热会 (填“偏大”、“偏小”或“无影响”)。
(1)1/2H2SO4(aq)+ NaOH (aq)="=" 1/2Na2SO4(aq)+H2O(1);△H=-57.3kJ·mol-1(2分)
(2)环形玻璃搅拌棒(1分)偏大(1分)
(3)-51.8 kJ/mol(2分)
(4)偏小(1分)
