(共15分) I(9分)写出下列热化学反应方程式
(1)N2 (g)与H2(g)反应生成1molNH3(g),放出46.1KJ热量。
(2)1molC2H5OH(l)完全燃烧生成CO2(g)和H2O(l),放出1366.8KJ热量。
(3)1molC(石墨)与适量H2O(g)反应吸收131.3KJ热量
II .(6分)(1)化学反应中均伴随着能量的变化,化学键的断裂和形成是发生能量变化的主要原因。生成物中化学键形成时会__________能量(填“放出”或“吸收”);如果一个化学反应,化学键断裂时的能量变化大于化学键形成时的能量变化,则该反应属于_________反应;如果一个化学反应,反应物的总能量和生成物的总能量有如图所示的关系,则该反应属于__________反应。

(2)不同形式的能量可以相互转换,如:化学能和电能、热能之间的相互转换。如图是一个原电池工作原理的示意图。试回答:
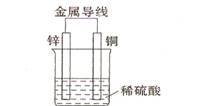
①从能量角度看,它属于____________能转化为____________能;
②装置中Zn为____________极。
(18分)I(1)1/2N2(g)+3/2H2(g)=NH3(g) △H=-46.1 kJ·mol-1
(2)C2H5OH(g)+3O2(g)==2CO2(g)+3H2O(l);△H=-1366.8 kJ·mol-1
(3)C(石墨s)+H2O(g)="=" CO(g)+ H2(g);△H=131.3 kJ·mol-1(每空2分)
II(1)放出 吸热 放热 (2)①化学 电 ②负 (每空1分)
I热化学方程式的书写,注意物质的状态,计量数,吸放热与△H的 “+”“ -”号间关系。
(1)1/2N2(g)+3/2H2(g)=NH3(g) △H=-46.1 kJ·mol-1
(2)C2H5OH(g)+3O2(g)==2CO2(g)+3H2O(l);△H=-1366.8 kJ·mol-1
(3)C(石墨s)+H2O(g)="=" CO(g)+ H2(g);△H=131.3 kJ·mol-1
II(1)放出 吸热 放热 (2)①化学 电 ②Zn为活泼金属,作负极。
