问题
实验题
(12分)中和热的测定实验的关键是要比较准确地配制一定的物质的量浓度的溶液,量热器要尽量做到绝热;在量热的过程中要尽量避免热量的散失,要求比较准确地测量出反应前后溶液温度的变化。回答下列问题:
(1)中学化学实验中的中和热的测定所需的玻璃仪器有:_________ ___________,在大小烧杯之间填满碎泡沫(或纸条)其作用是_____ _______________。
(2)该实验可用0.60 mol·L-1 HCl和0.65 mol·L-1的NaOH溶液各50 mL。NaOH的浓度大于HCl的浓度作用是___ __。当室温低于10℃时进行,对实验结果会造成较大的误差其原因是_________。
(3)若上述HCl、NaOH溶液的密度都近似为1 g/cm3,中和后生成的溶液的比热容C=4.18 J/(g·℃),则该中和反应放出热量为_____________kJ(填表达式),ΔH="___________" kJ/mol(填表达式)。
答案
(1)大烧杯、小烧杯、温度计、量筒、保温、隔热、减少实验过程中热量的损失
(2)保证盐酸完全被中和 散热太快
(3)0.418(t2-t1)-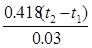
中和热的测定关键是在量热的过程中要尽量避免热量的散失。具体解答见答案。
