There was a famous church in London. It was 110 meters high. The center of the church was like a bell. The ceiling of the church was painted by a famous artist. A platform (平台)was built so that the artist could put up the ladder on it and paint the ceiling. The platform was about 70 meters over the ground.
One day the artist was working hard and had nearly finished one popular corner of the ceiling. Also on the platform was his assistant, who was mixing paints. The artist stepped back so that he could see how his work was progressing. To have a better look at his painting, he stepped back again. Suddenly, his assistant shouted, picked up a small bowl of paint and started to paint the artist’s work on the top. The artist was very angry and rushed forwards to stop him.
“What do you think you are doing?” he shouted angrily. “Are you mad?” His assistant replied, “I saw you walking backwards to have a better look at your painting. But you didn’t notice that you had reached the very edge of the platform. You were in great danger. I wanted to make you move forwards. If I had not made you run forwards, you would have fallen backwards off the edge of the platform.
小题1:The story happened in ______.
A.America
B.France
C.German
D.England小题2:Why did the artist step back again?
A.To see how his work was progressing.
B.To have a better look at his painting.
C.To paint a bow in his painting.
D.To pour some paint on his painting.小题3:What did the assistant do to stop the artist falling off the platform?
A.He painted a famous painting himself.
B.He ran forwards to stop him.
C.He shouted to the artist and painted his painting.
D.He stopped mixing the paints.小题4:Which picture shows the same situation as the underlined sentence in Paragraph2?
Note: a: artist b: assistant c: painting ←: the direction of the movement.
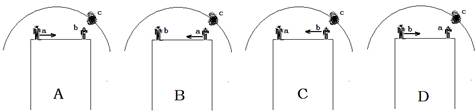
小题5:After reading the passage, what do you think the artist would probably do?
A.He would be angry with what the assistant had done.
B.He would punish the assistant for having destroyed his painting.
C.He would be thankful to be the assistant for having saved his life.
D.He wouldn’t allow the assistant to work with him any more.
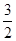 O2(g)===CO2(g)+2H2O(l) ΔH=-764.7 kJ·mol-1
O2(g)===CO2(g)+2H2O(l) ΔH=-764.7 kJ·mol-1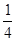 (d+6a+5c-12b)
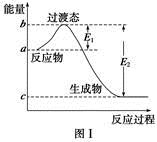
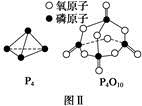
(d+6a+5c-12b) P4O10,结合图Ⅱ中白磷及其完全燃烧产物的结构,根据“反应热=反应物键能总和-生成物键能总和”与燃烧热概念可得等式:6a+5c-(4x+12b)=-d,据此可得 x=
P4O10,结合图Ⅱ中白磷及其完全燃烧产物的结构,根据“反应热=反应物键能总和-生成物键能总和”与燃烧热概念可得等式:6a+5c-(4x+12b)=-d,据此可得 x=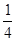 (d+6a+5c-12b)。
(d+6a+5c-12b)。
