(10分)(1)常温下,某水溶液M中存在的离子有:Na+、A2-、HA-、H+、OH-,存在的分子有H2O、H2A。根据题意回答下列问题:
①写出酸H2A的电离方程式__________________________。
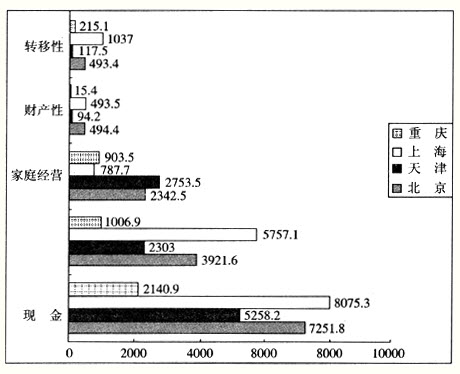
②若溶液M由2 mol·L-1NaHA溶液与2mol·L-1NaOH溶液等体积混合而得,则溶液M的pH ____7 (填“>”、“<”或“=”);溶液M中各微粒的浓度关系正确的是 。
A.c(Na+)>c(A2-)>c(OH-) >c(H+)
B. c(HA-) +c(H2A) +c(H+)=c(OH-)
C.c(A2-)+c(HA-) +c(H2A)=1 mol·L-1
D. c(A2-)+c(HA-)+c(OH-)=c(Na+)+c(H+)
(2)室温时,氢氧化钙的溶度积KSP =4.7×10-6, 室温时将9 mL0.02 mol·L—1的氯化钙溶液与1 mL pH=13的氢氧化钠溶液混合后(溶液体积可直接加和),溶液中___ 沉淀析出(填“有”或“无”)。
(3) 某校课外活动小组为测定已部分脱水的生石膏的组成(xCaSO4·yH2O),做如下实验:将固体加热,经测量剩余固体质量随时间变化如图所示。

则x:y= 。t2~t3时间段固体的化学式为 。t5~t6时间段固体质量减轻的原因是产生了两种气体,其中一种能使品红溶液褪色。则该时间所发生反应的化学方程式为 。
(10分)

(2)无(1分)
(3) 2:3(1分)
2CaSO4·H2O或CaSO4·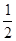 H2O(2分)
H2O(2分)
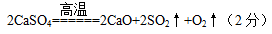
(1)①溶液中存在H2A则说明H2A为弱电解质,所以电离方程式为: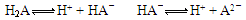
②溶液M由2 mol·L-1NaHA溶液与2mol·L-1NaOH溶液等体积混合,则混合后溶液中的溶质为Na2A,属于强碱弱酸盐,溶液呈碱性。溶液M中各微粒的浓度关系为:c(Na+)>c(A2-)>c(OH-) >c(H+)遵循电荷守恒;c(A2-)+c(HA-) +c(H2A)=1 mol·L-1 物料守恒;
(2)9 mL0.02 mol·L—1的氯化钙溶液与1 mL pH=13的氢氧化钠溶液混合后,反应前溶液中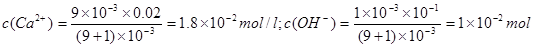
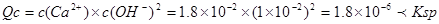 ,即可得溶液中无沉淀析出;
,即可得溶液中无沉淀析出;
(3)根据t5~t6时间段固体质量减轻的原因是产生了两种气体,其中一种能使品红溶液褪色,可知D点成分为CaSO4固体,由图数据可知

即x:y=2:3;t5~t6所发生反应的化学方程式为。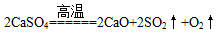
t2~t3时间段

2×136+3×18=326 18x
3.26 3.26-2.90=0.36g
解得X=2
即t2~t3时间段固体的化学式为2CaSO4·H2O或CaSO4·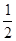 H2O
H2O