问题
选择题
常温下,用0.1000mol/LNaOH溶液分别滴定25.00mL0.1000mol/L盐酸溶液和
25.00mL0.1000mol/LCH3COOH溶液,滴定过程中pH变化曲线如下图所示。下列判断不正确的是
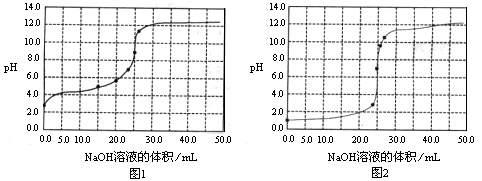
A.滴定盐酸的pH变化曲线为图2
B.在滴定CH3COOH溶液的过程中,始终都有c(Na+)+ c(H+)=c(CH3COO–)+c(OH–)
C.滴定CH3COOH溶液的过程中,当滴加12.5mLNaOH溶液时,溶液中离子浓度由大到小的顺序为c(CH3COO–)>c(Na+)>c(H+)>c(OH–)
D.当c(Na+)=c(CH3COO–)+ c(CH3COOH)时,溶液的pH<7
答案
答案:D
题目分析:A、盐酸是强酸,0.1mol/L的盐酸溶液中pH=1,所以滴定盐酸的pH变化曲线为图2,A正确;B、根据电荷守恒可知,在滴定CH3COOH溶液的过程中,始终都有c(Na+)+ c(H+)=c(CH3COO–)+c(OH–),B正确;C、根据图①可知,滴定CH3COOH溶液的过程中,当滴加12.5mLNaOH溶液时,溶液显酸性,这说明醋酸的电离程度大于醋酸钠的水解程度,则溶液中离子浓度由大到小的顺序为c(CH3COO–)>c(Na+)>c(H+)>c(OH–),C正确;D、根据物料守恒可知,当醋酸与氢氧化钠恰好反应生成醋酸钠时,溶液中c(Na+)=c(CH3COO–)+ c(CH3COOH),此时溶液的pH>7,D不正确,答案选D。