问题
选择题
化学反应N2+3H2 =2NH3的能量变化如下图所示,该反应的热化学方程式是
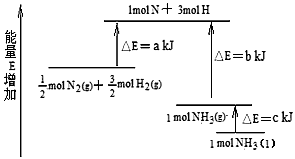
A.N2(g)+3H2(g)=2NH3 (l);⊿H = 2(a-b-c)kJ·mol-1
B.N2(g)+3H2(g)=2NH3(g) ;⊿H = 2(b-a)kJ·mol-1
C.1/2N2(g)+3/2H2(g)=NH3(l) ;⊿H = (b+c-a)kJ·mol-1
D.1/2N2(g)+3/2H2(g)=NH3(g) ;⊿H = (a+b)kJ·mol-1
答案
答案:A
