问题
选择题
已知:(1)Zn(s)+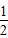 O2(g)═ZnO(s),△H=-348.3kJ/mol(2)2Ag(s)+ O2(g)═ZnO(s),△H=-348.3kJ/mol(2)2Ag(s)+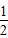 O2(g)═Ag2O(s),△H=-31.0kJ/mol O2(g)═Ag2O(s),△H=-31.0kJ/mol则Zn与Ag2O反应生成ZnO和Ag的热化学方程式为( ) A.Zn(s)+Ag2O(s)═ZnO(s)+2Ag(s),△H=317.3kJ/mol B.Zn+Ag2O═ZnO+2Ag,△H=317.3kJ/mol C.Zn(s)+Ag2O(s)═ZnO(s)+2Ag(s),△H=-317.3kJ/mol D.2Zn(s)+2Ag2O(s)═2ZnO(s)+4Ag(s),△H=-317.3kJ |
答案
答案:C
