含硫化合物在工业生产中有广泛的用途。
(1)SO2可用于工业生产SO3。
①在一定条件下,每生成8g SO3气体,放热9.83kJ。该反应的热化学方程式为________。
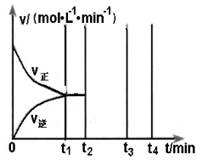
②在500℃,催化剂存在的条件下,向容积为1L的甲、乙两个密闭容器中均充入 2 mol SO2和1 mol O2。甲保持压强不变,乙保持容积不变,充分反应后均达到平衡。
I.平衡时,两容器中SO3体积分数的关系为:甲_______乙(填“>”、“<”或“ =”)。
II.若乙在t1 min时达到平衡,此时测得容器乙中SO2的转化率为90%,则该
反应的平衡常数为_______;保持温度不变,t2 min时再向该容器中充入1 mol SO2和1 mol SO3,t3min时达到新平衡。请在下图中画出t2~t4 min内正逆反应速率的变化曲线(曲线上必须标明V正、V逆 )
(2)硫酸镁晶体(MgSO4·7H2O )在制革、医药等领域均有广泛用途。4.92g硫酸镁晶体受热脱水过程的热重曲线(固体质量随温度变化的曲线)如右图所示。
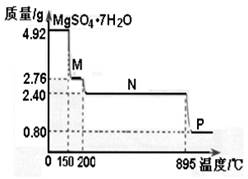
①固体M的化学式为__________。
②硫酸镁晶体受热失去结晶水的过程分为_________个阶段。
③N转化成P时,同时生成另一种氧化物,该反应的化学方程式为_________。
(1)①2SO2(g)+O2(g)⇌2 SO3(g)△H="-196.6" kJ/mol
②I > II 810 L/mol
(2)①MgSO4•H2O ② 2 ③MgSO4 MgO+SO3↑
MgO+SO3↑
(1)①在一定条件下,每生成8gSO3的物质的量为0.1mol,放热9.83kJ,则每生成1mol三氧化硫反应放热98.3KJ,所以该反应的热化学方程式为:2SO2(g)+O2(g)⇌2SO3(g)△H="-196.6" kJ/mol。
②I.2SO2(g)+O2(g)⇌2SO3(g)正反应是体积缩小的反应,在500℃,催化剂存在的条件下,向容积为1L的甲、乙两个密闭容器中均充入2mol SO2和1mol O2,起始时甲、乙两容器体积相同,甲保持压强不变,乙保持容积不变,充分反应后均达到平衡,甲为了保持压强不变,容器的体积不断减小,这一过程相当于到达平衡后的乙对容器加压,加压平衡正移.因此,达平衡时,甲容器中SO3体积分数大于乙容器中的三氧化硫,故答案为>;
II.乙在t1min时达到平衡,此时测得容器乙中的转SO2化率为90%,则2mol SO2和1mol O2在1L密闭容器在t1min内各物质浓度发生的变化如下:
2SO2(g)+O2(g)═2SO3(g)
起始浓度(mol/L) 2 1 0
变化浓度(mol/L)1.8 0.9 1.8
平衡浓度(mol/L)0.2 0.1 1.8
该反应的平衡常数为:k= C2(SO3)/C2(SO2)×C(O2)=(1.8mol/L)2/(0.2mol/L)2×(0.1mol/L)="810" L/mol。保持温度不变t2min时,化学平衡常数不变,再向该容器中充入1molSO2和1mol SO3,反应物SO2和生成物SO3,浓度在t2时都瞬间增加,所以t2时瞬间反应速率增大,在1L密闭容器在t2min内各物质浓度如下:
2SO2(g)+O2(g)═2SO3(g),
起始浓度(mol/L) 1.2 0.1 2.8
此时浓度商为:QC= C2(SO3)/C2(SO2)×C(O2)=(2.8mol/L)2/(1.2mol/L)2×(0.1mol/L)<K="810" L/mol,平衡向正反应方向移动,t3min时达到新平衡,图象如图所示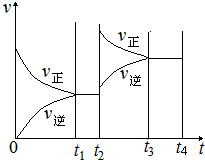
(2)①依据图象分析在150℃时固体质量变为2.76g,硫酸镁晶体加热失去结晶水,质量减小,设失去结晶水x个,则4.92g MgSO4•7H2O样品物质的量=4.92g÷246g/mol=0.02mol,
MgSO4•7H2O MgSO4•(7-x)H2O+xH2O
MgSO4•(7-x)H2O+xH2O
0.02mol 0.02xmol
0.02xmol×18g/mol=4.92g-2.76g=2.16g
x=6 所以加热到150℃时固体为MgSO4•H2O
②依据图象分析在200℃时固体质量变为2.40g,硫酸镁晶体加热失去结晶水,质量减小,设失去结晶水Y个,
MgSO4•7H2O  MgSO4•(7-Y)H2O+YH2O
MgSO4•(7-Y)H2O+YH2O
0.02mol 0.02Ymol
0.02Ymol×18g/mol=4.92g-2.40g=2.52g, x=7
所以加热到200℃时固体为MgSO4,所以硫酸镁晶体受热失去结晶水的过程分为两个阶段。故答案为2;
③硫酸镁晶体(MgSO4•7H2O)加热到200℃N时固体为硫酸镁,硫酸镁只含三种元素,N转化成P时,同时生成另一种氧化物,另一种氧化物只能是硫的氧化物,因镁的化合价未变,所以氧化物为三氧化硫,从图象中可知P时,固体质量为0.8克,氧化镁的物质的量为0.8g÷40g/mol=0.02mol,N转化成P时减少的固体质量2.40g-0.8=1.6g,硫的物质的量等于硫酸镁晶体的物质的量为0.02mol,所以氧元素的质量为1.6g-0.02mol×32g/mol=0.96g,物质的量为0.96g÷6g/mol=0.06mol,所以硫与氧的物质的量比为:0.02:0.06=1:3,进一步验证为三氧化硫,所以反应为:MgSO4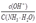 MgO+SO3↑。
MgO+SO3↑。
