问题
选择题
已知:H-H键的键能为436 kJ/mol,N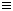 N键的键能为945 kJ/mol,N-H键的键能为391 kJ/mol。则下列有关工业合成氨反应的热化学方程式正确的是 [ ]
N键的键能为945 kJ/mol,N-H键的键能为391 kJ/mol。则下列有关工业合成氨反应的热化学方程式正确的是 [ ]
A. N2(g)+3H2(g)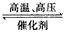 2NH3(g) △H = -93 kJ/mol
2NH3(g) △H = -93 kJ/mol
B. N2(g)+3H2(g)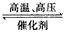 2NH3(g) △H = +1471 kJ/mol
2NH3(g) △H = +1471 kJ/mol
C. N2(g)+3H2(g)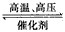 2NH3(g) △H = +93 kJ/mol
2NH3(g) △H = +93 kJ/mol
D. N2(g)+3H2(g)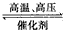 2NH3(g) △H= -1471 kJ/mol
2NH3(g) △H= -1471 kJ/mol
答案
答案:A
