问题
选择题
化学反应N2+3H2=2NH3的能量变化如图所示,该反应的热化学方程式是
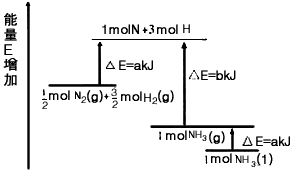
A. N2(g)+3H2(g)=2NH3(1); △H=2(a-b-c)kJ·mol-1
B. N2(g)+3H2(g)=2NH3(g); △H=2(b-a)kJ·mol-1
C. 1/2N2(g)+3/2H2(g)=NH3(1); △H=(h+c-a)kJ·mol-1
D. 1/2N2(g)+3/2H2(g)=NH3(g); △H=(a+b)kJ·mol-1
答案
答案:A
