问题
填空题
发射卫星用N2H4作燃料,NO2作氧化剂,两者反应生成N2和水蒸气,
已知:N2(g)+O2(g)=2NO2(g)△H1=+67.7kJ/mol
N2H4(g)+O2(g)=N2(g)+2H2O(g)△H2=-534kJ/mol
试写出N2H4与 NO2反应的热化学方程式______.
答案
①N2(g)+O2(g)=2NO2(g)△H1=+67.7kJ/mol
②N2H4(g)+O2(g)=N2(g)+2H2O(g)△H2=-534kJ/mol
依据盖斯定律:②×2-①得到:2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g)△H=-1135.7KJ/mol;
故答案为:2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g)△H=-1135.7KJ/mol.

 bZ(g);△H<0。如图是容器中X、Z的物质的量浓度随时间变化的曲线。下列说法正确的是( )
bZ(g);△H<0。如图是容器中X、Z的物质的量浓度随时间变化的曲线。下列说法正确的是( )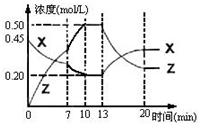
 0~10min容器内气体的压强逐渐增大
0~10min容器内气体的压强逐渐增大