第二节:书面表达(满分25分)
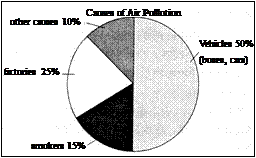
请根据右图表提示,以 “ Air pollution ” 为题,分析造成某城市空气污染的原因,并就如何解决这一问题提出一两条可行性建议。
注意:
1. 词数要求:120左右,开头已写好,不计入总词数。
2. 参考词汇:车辆—vehicle,有毒的—poisonous,因素—factor
Air pollution
With the development of industry, air pollution is getting more and more serious.
______________________________________________________________________________________________________________________________________________________________
One possible version
Air pollution
With the development of industry, air pollution is getting more and more serious. In this city, many people suffer from different kinds of illness because of air pollution.
What is the air pollution caused by ? About half of the problem is caused by vehicles. There are more and more cars and buses on the roads every day, which give off poisonous gases. About 30% of air pollution is caused by factories. Another factor is smoking. Smoking does harm to others’ health as well as to that of the smokers themselves. About 10% of air pollution is caused by others.
We must take measures to fight against this pollution. New fuel can be used to re place gas. We can plant more tress. If everybody realizes the importance of the environment and does something to stop pollution, the problem will be greatly reduced. (129 words )
place gas. We can plant more tress. If everybody realizes the importance of the environment and does something to stop pollution, the problem will be greatly reduced. (129 words )
