氮及其化合物与人类各方面有着密切的联系。Ⅰ现有一支15mL的试管,充满NO倒置于水槽中,向试管中缓缓通入一定量氧气,当试管内液面稳定时,剩余气体3mL。则通入氧气的体积可能为 mL。
Ⅱ目前,消除氮氧化物污染有多种方法。
(1)用CH4催化还原氮氧化物可以消除氮氧化物的污染。已知:
①CH4(g)+4NO2(g)= 4NO(g)+CO2(g)+2H2O(g) △H="-574kJ/mol"
②CH4(g)+4NO(g)= 2N2(g)+CO2(g)+2H2O(g) △H="-1160kJ/mol"
③H2O(g)= H2O(l) △H=-44kJ/mol
写出CH4(g)与NO2(g)反应生成N2(g) 、CO2(g)和H2O(l)的热化学方程式 。
(2)用活性炭还原法处理氮氧化物,有关反应为:
某研究小组向恒容密闭容器加入一定量的活性炭和NO,恒温(T0C)条件下反应,反应进行到不同时间测得各物质的浓度如下:
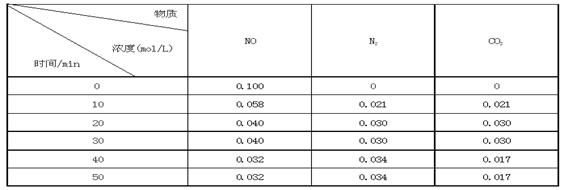
①不能作为判断反应达到化学平衡状态的依据是_______ ;(选填字母代号)
A.容器内CO2的浓度保持不变
B.v正(N2)="2" v正(NO)
C.容器内压强保持不变
D.混合气体的密度保持不变
E.混合气体的平均相对分子质量保持不变
②前20分钟,平均反应速率v(NO)= 。v(NO)=(0.1- 0.04)/ 20 = 0.003mol·L-1· min-1
③在T0C时,该反应的平衡常数为_______(保留两位小数);
④在30 min,改变某一条件反应重新达到平衡,则改变的条件是_______ 。
(3)科学家正在研究利用催化技术将超音速飞机尾气中的NO和CO转变成CO2和N2,其反应为:

研究表明:在使用等质量催化剂时,增大催化剂的比表面积可提高化学反应速率。为了分别验证温度、催化剂的比表面积对化学反应速率的影响规律,某同学设计了三组实验,部分实验条件已经填在下表中。
| 实验编号 | T(0C) | NO初始浓度 (mol/L) | CO初始浓度 (mol/L) | 催化剂的比 表面积(m2/g) |
| Ⅰ | 280 | 1.20×10-3 | 5.80×10-3 | 82 |
| Ⅱ | a | b | c | 124 |
| Ⅲ | 350 | d | e | 124 |
上表中:a=_______,b=________,e=________ 。
Ⅰ:9ml或14.25ml
Ⅱ:(1) CH4(g)+2NO2(g)= N2(g)+CO2(g)+2H2O(l) △H="-955" kJ/mol
(2)①BC ②0.003mol·L-1· min-1 ③0.56 ④减小CO2 的浓度
(3)a=280℃ b= 1.20×10-3 e=5.80×10-3
题目分析:(Ⅰ)往充满NO的试管中充入氧气并倒扣在水槽,发生的反应是4NO + 3O2 +2H2O = 4HNO3,最终剩余的气体可能是NO或O2;所以设氧气体积总共为X,
则①剩余氧气3ml时,4NO + 3O2 +2H2O = 4HNO3 ②剩余NO 3ml时,4NO + 3O2 +2H2O = 4HNO3
4: 3 4 :3
15ml X 15ml X
X="15×3/4" + 3 =14.25ml X=(15-3)×3/4 =9ml
(Ⅱ) (1) 据已知方程式可知目标方程式可以由方程式(①+②+③×4)÷2 得
CH4(g)+2NO2(g)= N2(g)+CO2(g)+2H2O(l),而△H=(△H1+△H2+4×△H3)÷2=" -955" kJ/mol
(2)在反应C(s)+2NO(g)= N2(g)+CO2(g)中,要判断是否达到平衡,要找变量,变量不变则达到平衡。容器内CO2的浓度是变量,保持不变时说明达到平衡,A正确;v正(N2)="2" v正(NO)表示的都是正反应的速率关系,不能说明正逆反应速率相等,所以不能判断平衡,B错误;反应方程式的前后系数相等,所以容器内压强随着反应保持不变,所以不能判断平衡,C错误;密闭容器是恒容,容器内总质量不变,但由于有固体参加,混合气体的质量是个变量,据ρ=m气/V知,当气体质量不变时,混合气体的密度保持不变,此时反应达到平衡,D正确; 同样混合气体的质量是个变量,据M=m气/n气 知,M也是个变量,当M保持不变时达到平衡,E正确。故选BC。
(3)v(NO)=(0.1- 0.04)mol·L-1/ 20min = 0.003mol·L-1· min-1
据表格数据可知,当反应达到20min时,各物质浓度不再变化反应达到第一次平衡,平衡常数
K=C(N2)·C(CO2)/ C2(NO)=0.032/0.042 =0.56 ;反应在40min时,各物质浓度发生变化,且C(CO2)浓度减小最多,平衡往正反应方向移动,所以改变的条件是减小CO2 的浓度。
(4)实验要验证的是温度、催化剂的比表面积对化学反应速率的影响规律,所以对比试验要控制变量,所以a=280℃ b= 1.20×10-3 e=5.80×10-3 。
