问题
选择题
已知下列反应的热化学方程式为
(1)CH3COOH(l)+2O2(g)=2CO2(g)+2H2O(l) ΔH1=-870.3 kJ/mol
(2)C(s)+O2(g)=CO2(g) ΔH2=-393.5 kJ/mol
(3)H2(g)+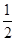 O2(g)=H2O(l) ΔH3=-285.8 kJ/mol
O2(g)=H2O(l) ΔH3=-285.8 kJ/mol
则反应2C(s)+2H2(g)+O2(g)=CH3COOH(l)的ΔH为( )
A.-488.3 kJ/mol
B.-191 kJ/mol
C.-476.8 kJ/mol
D.-1 549.6 kJ/mol
答案
答案:A
题目分析:(2)×2+(3)×2-(1)可得反应2C(s)+2H2(g)+O2(g)=CH3COOH(l) ΔH=2ΔH2+2ΔH3-ΔH1="-488.3" kJ/mol。选项为:A.
