问题
选择题
已知:Fe2O3(s)+3C(石墨)=2Fe(s)+3CO(g)ΔH=+489.0 kJ·mol-1①
CO(g)+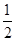 O2(g)=CO2(g)ΔH=-283.0 kJ·mol-1②
O2(g)=CO2(g)ΔH=-283.0 kJ·mol-1②
C(石墨)+O2(g)=CO2(g)ΔH=-393.5 kJ·mol-1③
则4Fe(s)+3O2(g)=2Fe2O3(s)的ΔH为( )
A.+1 164.1 kJ·mol-1
B.-1 641.0 kJ·mol-1
C.-259.7 kJ·mol-1
D.-519.4 kJ·mol-1
答案
答案:B
根据盖斯定律,将③×6-①×2-②×6得:4Fe(s)+3O2(g)=2Fe2O3(s),则ΔH=-393.5 kJ·mol-1×6-489.0 kJ·mol-1×2+283.0 kJ·mol-1×6=-1 641.0 kJ·mol-1。
