节能减排是当今社会的热门话题,研发混合动力汽车对于中国汽车业的未来具有重要的战略意义。混合动力汽车持续工作时间长,动力性好的优点,无污染、低噪声的好处,汽车的热效率可提高10%以上,废气排放可改善30%以上,某种混合动力汽车的动力系统由“1.6L汽油机十自动变速器十20kW十200V镍氢电池”组成。
①混合动力汽车所用的燃料之一是乙醇,lg乙醇完全燃烧生成CO2气体和液态H2O放出30.0kJ热量,写出乙醇燃烧的燃烧热的热化学方程式 。
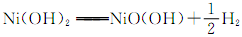 ②镍氢电池的使用可以减少对环境的污染,它采用储氢金属为负极,碱液NaOH为电解液,镍氢电池充电时发生反应 。其放电时的正极的电极反应方程式为 。
②镍氢电池的使用可以减少对环境的污染,它采用储氢金属为负极,碱液NaOH为电解液,镍氢电池充电时发生反应 。其放电时的正极的电极反应方程式为 。
③常温下,同浓度的Na2CO3溶液和NaHCO3溶液的pH都大于7,两者中哪种的pH更大,其原因是 。0.1mol·L-1 Na2CO3中阴离子浓度大小关系是 ,向0.1mol·L-1 NaHCO3溶液中滴入少量氢氧化钡溶液,则发生反应的离子方程式为: 。
(2)二氧化锰、锌是制备干电池的重要原料,工业上用软锰矿(含MnO2)和闪锌矿(含ZnS)
联合生产二氧化锰、锌的工艺如下:
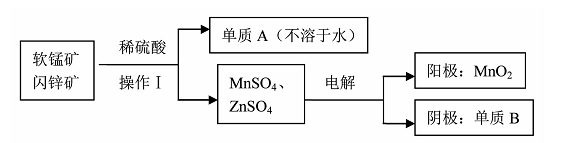
①操作Ⅰ需要的玻璃仪器是 。
②软锰矿(含MnO2)和闪锌矿与硫酸反应的化学方程式为 ,上述电解过程中,当阴极生成6.5g B时阳极生成的MnO2的质量为 。
③利用铝热反应原理,可以从软锰矿中提取锰,发生的化学方程式为 。
(1)①C2H5OH(l)+3O2(g)=2CO2(g) +3H2O(l);△H=-1380KJ/mol (2分)
② NiO(OH) + H2O+ e- = Ni(OH)3 + OH- ; (2分)
③Na2CO3,CO32- 水解比HCO3- 水解程度大,产生的OH- 更多,所以Na2CO3溶液的碱性比NaHCO3的大,PH大。 C(CO32-)﹥C(OH-)﹥C(HCO3-) (各1分)
Ba2+ + 2OH- + 2HCO3- =BaCO3↓+ CO32- + 2H2O (2分)
(2)① 漏斗、烧杯、玻棒 (写全得1分)
②MnO2 +ZnS +2H2SO4==MnSO4+ZnSO4+ S↓+2H2O; 8.7 g (各2分)
③ 3MnO2 + 4Al 2Al2O3 + 3Mn (2分)
2Al2O3 + 3Mn (2分)
题目分析:(1)①燃烧热指是1mol乙醇完全燃烧生成二氧化碳和液态水时放出的热量,故把1g换算成成46g,热量数值乘以46得乙醇的燃烧热;②放电时的反应为充电时的逆反应,放电时正极发生还原反应故NiO(OH)得电子发生还原反应生成Ni(OH)2;③根据两者水解程度的大小判断溶液pH的大小;水解是微弱的,碳酸钠溶液中主要存在的阴离子是碳酸根离子,碳酸根离子发生第一步水解生成碳酸氢根离子和氢氧根离子,碳酸氢根离子再发生水解生成碳酸分子和氢氧根离子,故C(CO32-)﹥C(OH-)﹥C(HCO3-);氢氧化钡溶液是少量的,可假设氢氧化钡的物质的量为1mol,2mol的OH-消耗2mol的HCO3-生成2mol的CO32-,1mol的钡离子只和1mol的碳酸根离子反应生成1mol的碳酸钡沉淀,故Ba2+ + 2OH- + 2HCO3- =BaCO3↓+ CO32- + 2H2O;(2)①操作Ⅰ是过滤,故需要漏斗做成过滤器、烧杯、玻璃棒引流;②反应物为MnO2和ZnS,产物MnSO4+ZnSO4,故二氧化锰发生了还原反应,发生氧化反应的是负二价的硫,氧化后生成不溶水的硫单质,故为MnO2 +ZnS +2H2SO4==MnSO4+ZnSO4+ S↓+2H2O;根据信息联合生产二氧化锰、锌,故阴极生成6.5g的锌,物质的量为0.1mol,转移电子的物质的量为0.2mol,当MnSO4得到0.2mol电子时生成0.1mol的MnO2,故质量为8.7g;③根据铝热反应得铝和二氧化锰高温下反应生成锰单质和氧化铝;
