问题
选择题
已知:Fe2O3(s) + 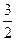 C(s) =
C(s) = 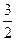 CO2(g) + 2 Fe(s) ΔΗ="234.1" kJ·mol-1
CO2(g) + 2 Fe(s) ΔΗ="234.1" kJ·mol-1
C(s) + O2(g) = CO2(g) ΔΗ=-393.5 kJ·mol-1
则2 Fe(s)+ 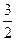 O2(g) = Fe2O3(s) 的ΔΗ是
O2(g) = Fe2O3(s) 的ΔΗ是
A.-824.4 kJ·mol-1
B.-627.6 kJ·mol-1
C.-744.7 kJ·mol-1
D.-169.4 kJ·mol-1
答案
A
 (2)=(1)就可得2 Fe(s)+
(2)=(1)就可得2 Fe(s)+  O2(g) = Fe2O3(s),则ΔΗ=
O2(g) = Fe2O3(s),则ΔΗ= ΔΗ2-ΔΗ1=-824.4 kJ·mol-1。
ΔΗ2-ΔΗ1=-824.4 kJ·mol-1。
