气化和液化是使煤变成清洁能源的有效途径。煤的气化的主要反应是:C+H2O(g) CO+H2,CO和H2的混合气体是合成多种有机物的原料气,研究由CO、H2合成有机物的化学称为一碳化学。下图是合成某些物质的路线图:
CO+H2,CO和H2的混合气体是合成多种有机物的原料气,研究由CO、H2合成有机物的化学称为一碳化学。下图是合成某些物质的路线图:
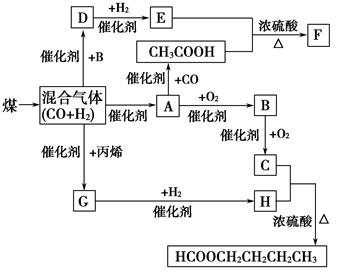
其中,D易溶于水,且与CH3COOH互为同分异构体,F分子中的碳原子数是D中的3倍,H经催化氧化可得到G。请回答下列问题:
(1)写出下列物质的结构简式:A: ,H: ,指出A和H的关系 。
(2)利用合成气(H2+CO)生产汽油、甲醇和氨等已经实现了工业化,合成气也可合成醛、酸、酯等多种产物,下列表述正确的是 。
①以合成气为原料的反应都是化合反应
②改变合成气中CO与H2的体积比,可得到不同的产物
③合成气的转化反应需在适当的温度和压强下通过催化完成
④从合成气出发生成气态烃或醇类有机物是实现“煤变油”的有效途径
⑤以合成气为原料的反应产物中不可能有烯烃或水
A.①②④
B.②③④
C.②④⑤
D.③④⑤(3)写出下列反应的化学方程式:
①CH3COOH+E―→F:____________________________________________。
②D与新制氢氧化铜悬浊液加热:___________________________________。
(1)CH3OH CH3CH2CH2CH2OH 同系物
(2)B
(3)①2CH3COOH+HOCH2CH2OH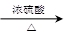 CH3COOCH2CH2OOCCH3+2H2O
CH3COOCH2CH2OOCCH3+2H2O
②HOCH2CHO+2Cu(OH)2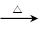 HOCH2COOH+Cu2O↓+2H2O
HOCH2COOH+Cu2O↓+2H2O
由D与CH3COOH互为同分异构体,故分子式为C2H4O2,D能和H2反应,E和CH3COOH发生酯化反应生成F,故D的结构简式为HO—CH2—CHO,E为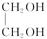 。由A
。由A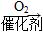 B
B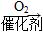 C衍变关系,可知A为CH3OH,C为HCOOH,故H应为CH3CH2CH2CH2OH。因H可催化氧化生成G,故G为CH3CH2CH2CHO。
C衍变关系,可知A为CH3OH,C为HCOOH,故H应为CH3CH2CH2CH2OH。因H可催化氧化生成G,故G为CH3CH2CH2CHO。

 0处语言错误,要求你在错误的地方增加、删除或修改某个单词。
0处语言错误,要求你在错误的地方增加、删除或修改某个单词。 completely free then, so I’ll to say “yes”. I’ll arrive in Bristol at around 8 pm in Friday
completely free then, so I’ll to say “yes”. I’ll arrive in Bristol at around 8 pm in Friday