按要求填空:
(1)拆开1mol H-H键,1mol N-H键,1mol N≡N键分别需要吸收的能量为436kJ,391kJ,946kJ.则理论上1mol N2生成NH3______热量(填:“吸收”或“放出”)______kJ;事实上,反应的热量总小于理论值,理由是______.
(2)X、Y两元素能形成XY2型化合物,XY2中共有38个电子,若XY2是离子化合物,其化学式是______;若XY2是共价化合物其结构式是______.
(3)第三周期内,X、Y两元素的原子序数之差为4,它们组成的XY型化合物,其电子式为______.
(4)某非金属X的最高正价为+m,它的最高价氧化物对应的水化物中有b个氧原子,则这种酸的化学式是______.
(5)X元素的最高正价和负价绝对值之差为6,Y元素和X元素原子的次外电子层上都有8个电子,X和Y形成的化合物YX2在水溶液中能电离出电子层结构相同的离子,用电子式表示该化合物的形成过程______.
反应热=吸收的总能量-放出的总能量,所以1mol N2生成NH3的反应热=946kJ+3×436kJ-2×3×391kJ=-92kJ,所以是放出热量,该反应是可逆反应,反应物不能完全转化为生成物,即充分反应的最终结果是达到最大限度,所以放出的热量总是小于理论计算值,
故答案为:放出;92;该反应是可逆反应,充分反应的最终结果是达到最大限度(即化学平衡状态),因此放出的热量总是小于理论计算值;
(2)XY2是离子化合物,X必然是2价的元素(不可能是4价的C和Si),由于X离子比Y离子多一个电子层,即X原子与Y原子相差3层电子,因此X只能是Ca,Y可以是F、O、C,但是只有CaF2恰好是38电子,满足题意,因此XY2是CaF2,如果XY2是共价化合物,则X必然是4价元素或者Be,通过电子计算,不难发现只有CS2满足要求.二硫化碳的结构式为:S=C=S.
故答案为:CaF2;S=C=S;
(3)X、Y两元素的原子序数之差为4,它们组成的XY型化合物,则可以看成A是+2价,第ⅡA族的镁;B是-2价,为第ⅥA族的硫,所组成的化合物是硫化镁,属于离子化合物,电子式为:Mg2++
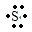
→
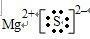
,
故答案为:Mg2++
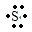
→
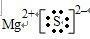
;
(4)氢元素化合价是+1价,氧元素的化合价是-2价,非金属X的最高正价为+m,设氢原子的数目是x,根据化合价守则,则x+m+(-2)×b=0,所以氢原子个数为2b-m,即酸的化学式是H(2b-m)XOb,故答案为:H(2b-m)XOb;
(5)根据题意,Y元素最高正价与最低负价的绝对值之差是6,则Y最高正价为+7,Y为Cl元素,再由Y元素与M元素形成离子化合物且在水中电离出电子层结构相同的离子,推出M为第四周期的金属元素,可能Ca元素,该化合物为CaCl2,属于离子化合物,CaCl2的形成过程可以表示为:
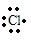
+Ca2++
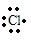
→
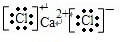
,
故答案为:
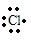
+Ca2++
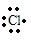
→
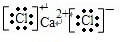
.
