问题
填空题
金属铜和浓硝酸、稀硝酸反应的方程式如下:
Cu+4HNO3(浓)=Cu(NO3)2+2NO2↑+2H2O
3Cu+8HNO3(稀)=3Cu(NO3)2+2NO↑+4H2O 工业上用铜屑和浓硝酸为原料制取硝酸铜,在实际生产中,需把浓硝酸用等体积水稀释。试简答:
(1)用稀硝酸而不用浓硝酸的原因:
(2)从经济效益和环保角度考虑、设计制取硝酸铜的最适宜方法,用化学方程式表示为: 。
答案
(1)制备等质量的硝酸铜时消耗的稀硝酸比浓硝酸少(或相同质量的稀硝酸比浓硝酸生产的硝酸铜多)
(2)2Cu+O2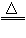 2CuO;CuO+2HNO3=Cu(NO3)2+H2O(或2Cu+O2+4HNO3=2Cu(NO3)2+2H2O)
2CuO;CuO+2HNO3=Cu(NO3)2+H2O(或2Cu+O2+4HNO3=2Cu(NO3)2+2H2O)
