在输液时,药液有时会从针口流出体外,为了及时发现,设计了一种报警装置,电路如图所示。M是贴在针口处的传感器,接触到药液时其电阻RM发生变化,导致S两端电压U增大,装置发出警报,此时
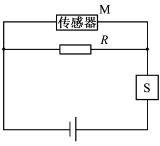
A. RM变大,且R越大,U增大越明显
B. RM变大,且R越小,U增大越明显
C. RM变小,且R越大,U增大越明显
D. RM变小,且R越小,U增大越明显
答案:C
根据“S两端电压U增大,装置发出警报”这一结果进行反推:说明电路里的电流在增大,再由闭合电路欧姆定律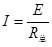 可知R总在减小,由此可推知传感器的电阻在变小,再由S与R、M的分压关系可讨论出R的大小对U的影响。
可知R总在减小,由此可推知传感器的电阻在变小,再由S与R、M的分压关系可讨论出R的大小对U的影响。
报警器两端的电压增大,则说明流过报警器的电流在增大,根据闭合电路欧姆定律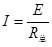 可知整个电路的总电阻R总在减小,则可得RM在变小,排除A、B答案;极限法:假设R很小,甚至为零,则传感器部分的电路被短路,故传感器RM的大小变化对S的电压就无影响,则R越大, U增大越明显,排除D项,C项正确。故答案为C。
可知整个电路的总电阻R总在减小,则可得RM在变小,排除A、B答案;极限法:假设R很小,甚至为零,则传感器部分的电路被短路,故传感器RM的大小变化对S的电压就无影响,则R越大, U增大越明显,排除D项,C项正确。故答案为C。
【考点定位】本题考查闭合电路欧姆定律与传感器、动态电路的结合,考查考生的分析、推理能力,要避免陷于对电路工作原理的纠缠。难度:较难。
