某同学设计如图装置,研究非金属元素性质变化规律.
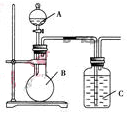
(1)已知硅酸(H2SiO3)是一种难溶于水的弱酸,呈白色。在化学反应中,一般地,强酸能制弱酸,如NaHCO3+HCl=NaCl+CO2↑+H2O,得出:HCl酸性强于H2CO3的酸性。现有硝酸溶液、碳酸钙、澄清石灰水、硅酸钠溶液,选择试剂用如图装置证明:酸性:HNO3>H2CO3>H2SiO3。
A中装试剂________,B中装试剂____________,C中装试剂____________。C中实验现象为____________;写出C中发生反应的离子方程式_____________________________。
(2)已知高锰酸钾在常温下与浓盐酸反应产生氯气,利用如图装置证明氯气氧化性强于碘单质的氧化性。则A中装浓盐酸,B中装入高锰酸钾粉末,C中装试剂________,C中现象________,写出离子方程式__________________。该实验装置有明显不足,请指出改进方法:_______________________________________。
(3)如果C中装饱和的氢硫酸溶液,A中装浓盐酸,B中装高锰酸钾溶液,反应开始后观察到现象是C中产生淡黄色沉淀,写出化学方程式______________________________;证明氯的非金属性比硫的非金属性________(填“强”或“弱”或“无法判断”)。
(1)硝酸溶液 碳酸钙 硅酸钠溶液 产生白色沉淀 CO2+SiO32-+H2O=H2SiO3↓+CO32-
(2)淀粉-KI溶液 溶液变蓝色 2I-+Cl2=I2+2Cl- 增加装有氢氧化钠溶液的尾气吸收装置
(3)Cl2+H2S= S↓+2HCl 强
题目分析:(1)利用强酸制备弱酸,可以根据所给试剂选择,硝酸和碳酸钙反应制备CO2,CO2通入硅酸钠溶液制备硅酸,产生白色沉淀;(2)利用氧化性强的制氧化性弱的,氯气和KI反应置换出碘单质,利用碘遇淀粉变蓝现象明显,选择淀粉碘化钾,氯气有毒,要注意尾气处理,以免污染环境。(3)氯气置换出硫,说明氯气氧化性强于硫,Cl2+H2S= S↓+2HCl。
