工业上利用软锰矿(主要成分为MnO2,同时含少量铁、铝等的化合物)制备硫酸锰的常见流程如下:

部分金属阳离子以氢氧化物形式完全沉淀时溶液的pH见下表:
| 沉淀物 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 10.4 |
(1)一氧化锰用硫酸酸浸时发生的主要反应的离子方程式为____________________。酸浸后加入MnO2将溶液中的Fe2+氧化成Fe3+,其目的是___________。
(2)滤渣A的成分除MnO2外,还有_______________。
(3)MnO2是制造碱性锌锰电池的基本原料,放电时负极的电极反应式为________。工业上以石墨为电极电解酸化的MnSO4溶液生产MnO2,阳极的电极反应式为_________,当阴极产生4.48L(标况)气体时,MnO2的理论产量为______g。
(4)锰的三种难溶化合物的溶度积:Ksp(MnCO3)=1.8×10-11,Ksp[Mn(OH)2]=1.9×10-13,Ksp(MnS)=2.0×10-13,则上述三种难溶物的饱和溶液中,Mn2+浓度由大到小的顺序是_______>_______>_______(填写化学式)。
(1)MnO+2H+=Mn2++H2O(2分) 确保铁元素在后续操作中完全转化为氢氧化物沉淀而除去(2分) (2)Al(OH)3、Fe(OH)3(2分)
(3)Zn+2OH--2e-=Zn(OH)2(2分) Mn2++2H2O-2e-=MnO2+4H+(3分) 17.4(3分)
(4)Mn(OH)2>MnCO3>MnS(3分)
题目分析:(1)一氧化锰用硫酸酸浸时生成硫酸锰和水,因此发生反应的离子方程式为MnO+2H+=Mn2++H2O;根据表中数据可知,氢氧化亚铁完全沉淀时的pH值是9.7,因此在pH等于5.4的条件下不能沉淀无法除去。而氢氧化铁完全沉淀时的pH值只有3.2,所以酸浸后加入MnO2将溶液中的Fe2+氧化成Fe3+的目的是保铁元素在后续操作中完全转化为氢氧化物沉淀而除去。
(2)根据表中数据可知,在pH等于5.4的条件下铝离子与铁离子已经完全转化为氢氧化物沉淀,因此滤渣A的成分除MnO2外,还有Al(OH)3、Fe(OH)3。
(3)MnO2是制造碱性锌锰电池的基本原料,而原电池中负极失去电子,发生氧化反应,该原电池中锌是负极,则放电时负极的电极反应式为Zn+2OH--2e-=Zn(OH)2;电解池中阳极失去电子,发生氧化反应,则工业上以石墨为电极电解酸化的MnSO4溶液生产MnO2,则锰离子在阳极放电,因此阳极的电极反应式为Mn2++2H2O-2e-=MnO2+4H+;电解池中阴极得到电子,发生还原反应,则阴极是溶液中的氢离子放电产生氢气。当阴极产生4.48L(标况)氢气,其物质的量是0.2mol,转移0.2mol×2=0.4mol电子,所以根据电子守恒可知,阳极生成二氧化锰的物质的量是0.2mol,则MnO2的理论产量为0.2mol×87g/mol=17.4g。
(4)锰的三种难溶化合物的溶度积:Ksp(MnCO3)=1.8×10-11,Ksp[Mn(OH)2]=1.9×10-13,Ksp(MnS)=2.0×10-13,由于碳酸锰与硫化锰的组成特点相似,因此溶液中的锰离子浓度与溶度积常数成正比,即溶度积常数越大,锰离子浓度越大,所以Mn2+浓度是MnCO3>MnS。根据溶度积常数可知,碳酸锰和氢氧化锰溶液中Mn2+浓度分别为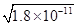 、
、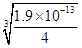 ,显然前者大于后者,所以上述三种难溶物的饱和溶液中,Mn2+浓度由大到小的顺序是Mn(OH)2>MnCO3>MnS。
,显然前者大于后者,所以上述三种难溶物的饱和溶液中,Mn2+浓度由大到小的顺序是Mn(OH)2>MnCO3>MnS。
