某银白色金属M在日常生产和生活中应用最广泛,M的某合金材料是建筑工程中不可缺少的建材;M在右图所示的反应中,可得到A、B、C、D四种产物,A为黑色固体,D的组成中M与Cl的原子个数比为1:3.
(1)A的化学式为(用M的真实元素符号表示,后同)______,A在物质分类中属于______(选填代号字母);a.单质b.酸c.碱d.盐e.有机物f.氧化物h.混合物
(2)物质B的化学式为______;M与Cu、(H)三者在金属活动性顺序中由强到弱的排序为:______;
(3)写出下列指定反应的化学方程式:反应③:______;反应④:______D+AgNO3反应:______.
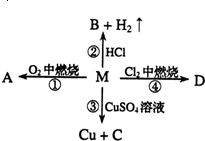
(1)由于M是日常生产和生活中应用最广泛的金属,所以M的化学式是Fe,A是铁燃烧的产物,所以A是四氧化三铁,该物质属于两种元素组成,其中一种是氧元素的化合物,所以是氧化物;
(2)铁与盐酸反应除生成氢气外还会生成氯化亚铁,化学式为FeCl2,依据金属活动性顺序表可知Fe与Cu、(H)三者在金属活动性顺序中由强到弱的排序为Fe、(H)、Cu;
(3)反应③是铁与硫酸铜生成铜与硫酸亚铁的反应,方程式为:Fe+CuSO4=FeSO4+Cu;反应④为铁与氯气的反应由于D的组成中M与Cl的原子个数比为1:3,所以生成物的化学式为FeCl3,该反应的方程式为:2Fe+3Cl2
2FeCl3;D+AgNO3反应是氯化铁与硝酸银的反应,其产物是氯化银沉淀和硝酸铁,方程式为:FeCl3+3AgNO3=3AgCl↓+Fe(NO3)3; 点燃 .
故答案为:(1)Fe;f;(2)FeCl2;Fe、(H)、Cu;(3)Fe+CuSO4=FeSO4+Cu;2Fe+3Cl2
2FeCl3;FeCl3+3AgNO3=3AgCl↓+Fe(NO3)3; 点燃 .
