问题
完形填空
| 完形填空 | ||||
| When I first ate in a western restaurant, I didn't know what I was supposed to do. Everything was l . I was used to _ 2 with chopsticks and a spoon, but I had to eat with a knife, a fork and a spoon. And I had, not just one set to 3 , but two or three of each. Questions 4 my mind. Was I supposed to begin with the largest or the smallest? Was I supposed to start at the outside and work in or the inside and work out? Was I supposed to hold the spoon in my left hand or my right hand? What I finally decide to do was just to 5 the others. | ||||
|
答案
1-5 B B A D C

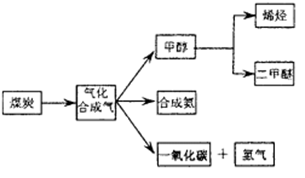
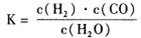
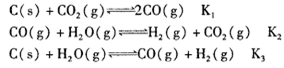
 , 该反应平衡常数随温度的变化如下:
, 该反应平衡常数随温度的变化如下:
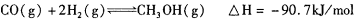
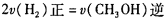
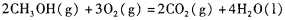 ,则负极的电极反应式为_______。
,则负极的电极反应式为_______。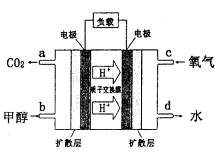
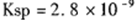 。 CaCl2溶液与Na2CO3溶液混合可形成CaCO3沉淀。现将CaCl2溶液与
。 CaCl2溶液与Na2CO3溶液混合可形成CaCO3沉淀。现将CaCl2溶液与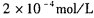 的Na2CO3溶液等体积混合,则生成沉淀时原CaCl2溶液的最小浓度为_______。
的Na2CO3溶液等体积混合,则生成沉淀时原CaCl2溶液的最小浓度为_______。