总分类账户与明细分类账户登记的原始依据和详细程度不同。()
参考答案:错
解析:
总分类账户与明细分类账户登记的原始依据相同但其详细程度不同。
总分类账户与明细分类账户登记的原始依据和详细程度不同。()
参考答案:错
解析:
总分类账户与明细分类账户登记的原始依据相同但其详细程度不同。
| 完形填空。 | ||||
| A 12-year-old boy saw something in a shop window that set his heart racing.But the price-five dollars-was far beyond Reuben Earle's wealth.Five dollars would buy almost a week's groceries for his family. But hearing the sound of hammering from a side street, Reuben had an idea. He ran towards the 1 and stopped at a construction site.People built their own homes in Bay Roberts, using nails _2_ in hessian sacks(麻袋)from a local factory.Sometimes the sacks were _3_, and Reuben knew he could sell them back to the 4_ for five cents a piece. Back home, he looked at his mother Dora and _5_.Sunlight from the window gilded her shoulder-length blonde hair.Slim and beautiful, she was the center of the home, the glue that held it together. Every day after school, Reuben walked down the town, _6_ the hessian nail bags.On the day the school closed for the summer, no student was more 7 than Reuben.Now he would have more time for his _8_. Then one day the _9_ had come. Reuben ran down Water Street to the store."Please, Mister.I have to sell the sacks now." The man took the sacks, 10 his pocket and put four coins in Reuben's hand.Reuben murmured (小 声说)a thank you and 11 , home. When he got home.Reuben 12 the tin can.He poured the coins out and began to 13 . He had enough. Then, he headed for the shop, "I have the 14 ," he told the owner. The man went to the window and took out Reuben's 15 . He wiped the dust off and gently wrapped it in brown paper.Then he placed it in Reuben's hands. Racing home, Reuben _16_ the front door."Here, Mum! Here'." He placed a small box in her work-roughened hands. She 17 it carefully. A jewel box appeared.Dora lifted the cover, 18 beginning to blur (模糊) her vision. Dora had never received such a 19 ; she had no jewellery except her wedding ring. 20 ,she smiled and gathered her son into her arms. | ||||
|
硫化碱法是工业上制备Na2S2O3的方法之一,反应原理为:2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2(该反应△H>0)。某研究小组在实验室用硫化碱法制备Na2S2O3·5H2O流程如下。
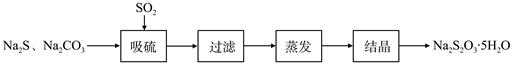
(1)吸硫装置如图所示。
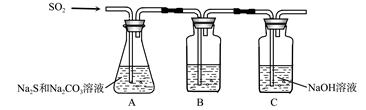
①装置B的作用是检验装置A中SO2的吸收效率,B中试剂是 ,表明SO2吸收效率低的实验现象是B中溶液 。
②为了使SO2尽可能吸收完全,在不改变A中溶液浓度、体积的条件下,除了及时搅拌反应物外,还可采取的合理措施是 、 。(写出两条)
(2)假设本实验所用的Na2CO3含少量NaCl、NaOH,设计实验方案进行检验。(室温时CaCO3饱和溶液的pH=10.2)
限选试剂及仪器:稀硝酸、AgNO3溶液、CaCl2溶液、Ca(NO3)2溶液、酚酞溶液、蒸馏水、pH计、烧杯、试管、滴管
| 序号 | 实验操作 | 预期现象 | 结论 |
| ① | 取少量样品于试管中,加入适量蒸馏水,充分振荡溶解,___________________。 | _______________ | 样品含NaCl |
| ② | 另取少量样品于烧杯中,加入适量蒸馏水,充分搅拌溶解,___________________。 | _______________ | 样品含NaOH |
(3)Na2S2O3溶液是定量实验中的常用试剂,测定其浓度的过程如下:准确称取a g KIO3(化学式量:214)固体配成溶液,加入过量KI固体和H2SO4溶液,滴加指示剂,用Na2S2O3溶液滴定至终点,消耗Na2S2O3溶液的体积为V mL。则c(Na2S2O3)=_________mol·L-1。(只列出算式,不作运算)
已知:Cr2O72-+6I-+14H+= 2Cr3++3I2+7H2O 2S2O32-+I2=S4O62-+2I-
(4)某同学第一步和第二步的操作都很规范,第三步滴速太慢,这样测得的Na2S2O3浓度可能 (填“无影响”、“偏低”或“偏高”),原因是 。