氨气与氟气在铜的催化作用下反应得到一种铵盐NH4F和一种含氮化合物A,A的分子结构呈三角锥形,分子中所有共价键之间的夹角都相等。
(1)A的化学式为 ,A的电子式为 。
(2)写出该反应的化学方程式 。
(3)现有1 mol混合气体充分反应后,测得生成物NH4F中的N元素比A中的N元素多2.8 g,试计算原混合气中NH3和F2的物质的量可能各是多少?
|
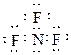 (2)4NH3+3F2
(2)4NH3+3F2 3NH4F+NF3
3NH4F+NF3∴n(NH3) =" 0.4" mol,n(F2) =" 0.6" mol 或n(NH3) =" 0.7" mol,n(F2) =" 0.3" mol |
28 |
Δm (N) |
0.3 |
0.4 |
4 |
3 |
4NH3+3F2  3NH4F+NF3 3NH4F+NF3 |
解: |
2.8 |
