课题小组为测定某石灰石样品中CaCO3的质量分数,取10g石灰石样品放在烧杯中,然后向其中逐渐加入稀盐酸,使之与样品充分反应(杂质不参与反应)。随反应进行,加入稀盐酸的体积与反应得到气体的质量呈下图所示关系。
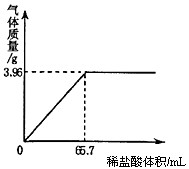
(1)样品中CaCO3的质量分数是多少?
(2)依题意,能否计算稀盐酸的质量分数?理由是什么?
解:设样品中CaCO3的质量为x。
CaCO3+2HCl==CaCl2+CO2↑+H2O
100 44
x 3.96g
100∶x=44∶3.96g
x=9g
(1)石灰石样品中CaCO3的质量分数=9g/10g×100%=90%
(2)不能,因为只知盐酸体积,不知盐酸密度,就不知盐酸在质量,无法求出盐酸的质量分数。
