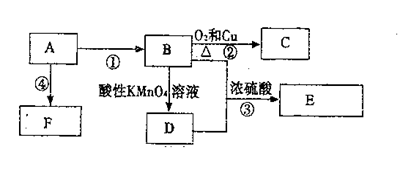
为了探究SO2与Na2O2的反应是否类似于CO2,甲同学设计了如图实验装置。
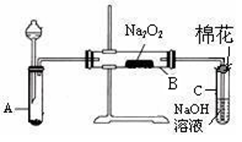
回答下列问题:
(1)制取SO2的合适试剂是 。
a.80%的H2SO4(aq) b.10%的H2SO4(aq)
c.Na2SO3(s) d.Na2SO3(aq)
(1)装置C中NaOH(aq)的作用是 。
(1)移开棉花,将带火星的木条放在C试管口,未见木条复燃。甲同学因此认为SO2与Na2O2的反应不同于CO2。请按甲同学的观点写出反应的化学方程式 。
(1)检验反应后B装置中生成物的方法是 。
(1)乙同学认为无论反应原理如何,最终都有O2产生。乙同学的理由是 。按照乙同学的观点,该装置还需作如下改进: 。(简要阐述,并指明必需的装置名称与试剂。)
(1)a、c(1分×2)
(2) 吸收多余的SO2,防止污染环境(1分×2)
(3)SO2+ Na2O2→ Na2SO4(2分)
(4)取少量B中固体于试管中,加盐酸至溶液呈酸性,再滴入氯化钡溶液,若有白色沉淀生成,则B中反应后的生成物里有Na2SO4(2分)
(5)A中生成的SO2气体中含有水蒸气(2分)。在A和B之间连接一个装有浓硫酸的洗气瓶(或其他合适的干燥装置)。合理给分。 (2分)
题目分析:(1)因SO2的溶解度较大,应选用浓度较大的H2SO4溶液;为了使反应顺利进行须选择Na2SO3固体与硫酸反应(CaSO3固体与硫酸反应生成的硫酸钙微溶于水).故答案为:bc(2)SO2有毒,直接排放到空气中污染环境.故答案为:吸收多余的SO2,防止污染环境(3)进入B装置的气体应该干燥,故应将②加在A和B之间.故答案为:②;将②加在A和B之间(4)木条不复燃,说明没有氧气生成,Na2O2有强氧化性,有SO2较强的还原性,产物应为Na2SO4,方程式为:SO2+Na2O2=Na2SO4.故答案为:SO2+Na2O2=Na2SO4
(5)在检验是否有Na2SO4生成时,要考虑到Na2SO3的干扰,因为SO32-有较强的还原性,可被过氧化钠氧化成SO42-.故答案为:不合理;如果固体中还有未反应的Na2O2,也能氧化亚硫酸钠为硫酸钠.(6)将Na2SO4转化成BaSO4沉淀,通过称量BaSO4沉淀的质量,计算含量,故应为acd。故答案为:acd