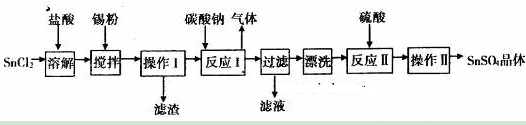
硫酸亚锡(SuSO4)是一种重要的硫酸盐,广泛应用于镀锡工业。某研究小组设计SnSO4制备路线如下:
查阅资料:
I.酸性条件下,锡在水溶液中有Sn2+、Sn4+两种主要存在形式,Sn2+易被氧化。
II.SnC12易水解生成碱式氯化亚锡[Sn(OH)Cl]。
回答下列问题:
(1)SnC12粉末需要加浓盐酸进行溶解,请用化学方程式说明原因___________________。
(2)在SnC12溶液中加入Sn粉的作用有两个:①调节溶液pH ②_________________。
(3)操作I中使用的玻璃仪器有___________________。
(4)该小组通过下列方法测定所用锡粉的纯度(杂质不参与反应):
①将试样溶于盐酸中,发生的反应为:Sn+2HCl═SnCl2+H2↑;
②加入过量的FeCl3,发生的反应为:SnCl2+ FeCl3= SnCl4+ FeCl2
③用已知浓度的K2Cr2O7滴定生成的Fe2+,发生的反应为:6FeCl2+K2Cr2O7+14HCl═6FeCl3+2KCl+2CrCl3+7H2O
滴定时,K2Cr2O7溶液应该装在____________(填“酸式”或“碱式”)滴定管中。
若取2.0g锡粉,经上述各步反应后,共用去0.100mol/L K2Cr2O7溶液40.00mL,锡粉中锡的质量分数是____________________。
(1)SnCl2+H2O Sn(OH)Cl+HCl(2)防止Sn2+被氧化(3)漏斗、玻璃棒、烧杯(4)酸式;71.4%
Sn(OH)Cl+HCl(2)防止Sn2+被氧化(3)漏斗、玻璃棒、烧杯(4)酸式;71.4%
题目分析:(1)SnCl2易水解生成碱式氯化亚锡,存在平衡SnCl2+H2O Sn(OH)Cl+HCl,加入盐酸,使该平衡向左移动,抑制Sn2+水解;(2)由题意知Sn2+易被氧化,加入Sn粉除调节溶液pH外,还防止Sn2+被氧化;(3)由流程图可知,操作Ⅰ是从溶液中得到含结晶水的晶体,只能采取蒸发浓缩、冷却结晶、过滤、洗涤得到,故用到的玻璃仪器为漏斗、玻璃棒、烧杯;(4)碱式滴定管的胶管与重铬酸钾溶液会有一定的氧化和吸附,影响浓度;令锡粉中锡的质量分数为x,则:
Sn(OH)Cl+HCl,加入盐酸,使该平衡向左移动,抑制Sn2+水解;(2)由题意知Sn2+易被氧化,加入Sn粉除调节溶液pH外,还防止Sn2+被氧化;(3)由流程图可知,操作Ⅰ是从溶液中得到含结晶水的晶体,只能采取蒸发浓缩、冷却结晶、过滤、洗涤得到,故用到的玻璃仪器为漏斗、玻璃棒、烧杯;(4)碱式滴定管的胶管与重铬酸钾溶液会有一定的氧化和吸附,影响浓度;令锡粉中锡的质量分数为x,则:
Sn~Sn2+~2Fe3+~2Fe2+~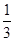 K2Cr2O7计算.
K2Cr2O7计算.
119g 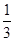 mol
mol
2.0g×x 0.100mol/L×0.040L
故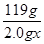 =
=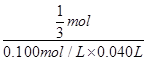
解得x=71.4%,
