请分析回答某同学在探究浓硫酸、稀硫酸、浓硝酸、稀硝酸分别与铜反应的实验中发现的有关问题。
Ⅰ.探究上述四种酸的氧化性相对强弱及其与铜反应的还原产物的性质
(1)分别向盛有等量铜片的四支试管中加入等体积的浓硫酸、稀硫酸、浓硝酸、稀硝酸,实验结果记录如下表:
| 酸 | 实验结果 | |
| a | 浓硫酸 | 加热后发生反应,产生无色刺激性气体 |
| b | 稀硫酸 | 加热也不发生反应 |
| c | 浓硝酸 | 不加热即发生反应,产生红棕色气体 |
| d | 稀硝酸 | 微热发生反应,产生无色气体 |
①由上表中实验结果,四种酸的氧化性由强到弱的顺序是 。
②由上表可知,铜与稀硫酸在加热条件下不反应,但若同时通入O2,铜片能逐渐溶解,溶液变为蓝色。写出该反应的化学方程式: 。
(2)先将铜与浓硫酸反应产生的气体X持续通入如图所示装置中,一段时间后再将铜与浓硝酸产生的气体Y也持续通入该装置中,则整个过程中可观察到的现象包括 (填字母)。

A.通入X气体后产生白色沉淀
B.通入X气体后溶液无明显现象
C.通入Y气体后产生沉淀
D.通入Y气体后沉淀溶解
E.通入Y气体后溶液中无明显现象
由此可得到的结论是 (填字母)。
A.硝酸的酸性比硫酸强
B.H2SO3的酸性比盐酸强
C.HNO3能氧化H2SO3(或SO2)
D.BaSO4既不溶于水也不溶于HNO3溶液
Ⅱ.如图是某同学探究铜与稀硝酸反应的还原产物的实验装置图,请回答下列问题:
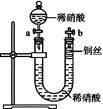
(1)写出铜与稀硝酸反应的离子方程式: 。
(2)已知装置气密性良好,请简述利用该装置证明铜与稀硝酸反应生成的气体产物是NO的操作过程: 。
Ⅰ.(1)①浓硝酸>稀硝酸>浓硫酸>稀硫酸
②2Cu+O2+2H2SO4 2CuSO4+2H2O
2CuSO4+2H2O
(2)BC CD
Ⅱ.(1)3Cu+8H++2
 3Cu2++2NO↑+4H2O
3Cu2++2NO↑+4H2O
(2)反应停止后打开b,如果看到无色气体变为红棕色,证明生成的气体是NO
根据反应条件“不加热即可发生反应”“微热发生反应”“加热后发生反应”“加热也不发生反应”可以判断氧化性由强到弱的顺序为浓硝酸>稀硝酸>浓硫酸>稀硫酸。铜、稀硫酸与O2反应的化学方程式为2Cu+O2+2H2SO4 2CuSO4+2H2O。铜与浓硫酸反应产生的气体X为SO2,通入BaCl2溶液中不反应,但再通入铜与浓硝酸产生的气体Y(NO2)以后,SO2被氧化为H2SO4,与BaCl2生成BaSO4沉淀。铜与稀硝酸反应产生的NO为无色气体,在U形管右上端聚集,反应一段时间停止后,打开b,如果看到无色气体变为红棕色,则证明生成的气体是NO。
2CuSO4+2H2O。铜与浓硫酸反应产生的气体X为SO2,通入BaCl2溶液中不反应,但再通入铜与浓硝酸产生的气体Y(NO2)以后,SO2被氧化为H2SO4,与BaCl2生成BaSO4沉淀。铜与稀硝酸反应产生的NO为无色气体,在U形管右上端聚集,反应一段时间停止后,打开b,如果看到无色气体变为红棕色,则证明生成的气体是NO。
