在我国青海湖地区有一种说法:冬天捞碱,夏天晒盐.这里的碱是指Na2CO3,盐是指NaCl.人们从盐湖中捞得的Na2CO3会含有少量的NaCl.
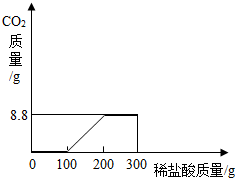
某研究性学习小组称取含NaCl的Na2CO3固体25.0g,将其配制成溶液,再向其中逐滴加入足量的7.3%的稀盐酸,使气体完全放出,共收集到8.8g CO2气体.
(1)计算原固体中Na2CO3的质量分数和消耗盐酸的总质量.
(2)下表为研究性学习小组向上述所配制的混合液中分三次逐滴加盐酸后,所记录的部分数据.
经查阅资料知:Na2CO3+HCl=NaCl+NaHCO3 (1)NaHCO3+HCl=NaCl+H2O+CO2↑(2)
已知:反应(1)完全后,反应(2)才开始.
①请完成表格中未填的部分.
| 实验次序 | 每次产生的CO2的质量(g) |
| 第一次先逐滴加盐酸100g | ______ |
| 第二次再逐滴加盐酸100g | 8.8 |
| 第三次再逐滴加盐酸100g | 0 |
(1)设原固体中Na2CO3的质量为x,消耗稀盐酸的质量为y
Na2CO3+2HCl=2NaCl+CO2↑+H2O
106 73 44
x y×7.3% 8.8g
=106 x
=73 y×7.3% 44 8.8g
x=21.2g y=200g
原固体中Na2CO3的质量分数=
×100%=84.8%21.2g 25.0g
答:原固体中Na2CO3的质量分数为84.8%,消耗盐酸的总质量为200g.
(2)设溶液中21.2g碳酸钠完成反应,需要稀盐酸质量为a,生成碳酸氢钠的质量为b
Na2CO3+HCl=NaCl+NaHCO3
106 36.5 84
21.2g a×7.3% b
=106 21.2g
=36.5 a×7.3% 84 b
a=100g b=16.8g
因此,第一次加入的100g稀盐酸恰好与碳酸钠溶液完全反应形成碳酸氢钠,该过程中没有二氧化碳放出;
设16.8g碳酸氢钠完成反应需要稀盐酸的质量为m,放出二氧化碳的质量为n
NaHCO3+HCl=NaCl+H2O+CO2↑
84 36.5 44
16.8g m×7.3% n
=84 16.8g
=36.5 m×7.3% 44 n
m=100g n=8.8g
因此第二次加入100g稀盐酸时开始放出二氧化碳且完全反应后碳酸氢钠反应完,最终放出二氧化碳质量为8.8g;所以第三次加入稀盐酸不再发生反应;
故答案为:①0;
②