为了预防碘缺乏症,国家规定每千克食盐中应含40~50mg碘酸钾。碘酸钾晶体有较高的稳定性,但在酸性溶液中,碘酸钾是一种较强的氧化剂,能跟某些还原剂作用生成碘;在碱性溶液中,碘酸钾能被氯气、次氯酸等强氧化剂氧化为更高价的碘的含氧酸盐。
【问题1】工业生产碘酸钾的流程如下:
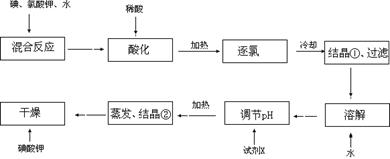
(1)碘、氯酸钾和水混合后的反应为(未配平):I2+KClO3+H2O→KH(IO3)2+KCl+Cl2↑。该方程式配平时,系数有多组,原因是 。
(2)X的化学式为 ;写出用试剂X调节pH的化学方程式: 。
(3)生产中,如果省去“酸化”、“逐氯”、“结晶①、过滤”这三步操作,直接用试剂X调整反应后溶液的pH,对生产碘酸钾有什么具体影响? 。
【问题2】已知:KIO3+5KI+3H2SO4→3K2SO4+3I2+3H2O; I2+2S2O32-→2I-+S4O62-。
(4)检验加碘食盐中的碘元素,学生甲利用碘酸钾与碘化钾在酸性条件下发生反应。用四氯化碳检验碘单质时,看到的明显现象有 。
(5)测定加碘食盐中碘的含量,学生乙设计的实验步骤如下:
a.准确称取w g食盐,加适量蒸馏水使其完全溶解;
b.用稀硫酸酸化所得溶液,加入过量KI溶液,使KIO3与KI反应完全;
c.以淀粉为指示剂,加入物质的量浓度为2.0×10-3mol·L-1的Na2S2O3溶液10.0mL恰好反应。
则加碘食盐样品中的碘元素含量是 mg/kg(以含w的代数式表示)。
(6)学生丙又对纯净的NaCl(不含KIO3)进行了下列实验:
| 操作步骤 | 实验现象 |
| 取1g纯净的NaCl,加3mL水配成溶液。 | 溶液无变化 |
| 滴入5滴淀粉溶液和1mL 0.1 mol·L-1 KI溶液,振荡。 | 溶液无变化 |
| 然后再滴入1滴1mol·L-1的H2SO4,振荡。 | 溶液变蓝色 |
①推测实验中产生蓝色现象的可能原因,用离子方程式表示 。
②根据学生丙的实验结果,请对学生乙的实验结果作出简要评价: 。
(1)有两种还原产物生成。
(2)KOH; KH(IO3)2+KOH=2KIO3+H2O
(3)反应产生的氯气跟KOH反应生成KClO, KClO能将KIO3氧化成KIO4从而不能得到碘酸钾。
(4)液体分层,下层液体呈现紫红色。
(5)1270/3W或 (423.33/W)。
(6)①4I-+4H++O2→2I2+2H2O;② 因加入了过量KI溶液,乙实验结果会偏大。
题目分析:
(1)碘、氯酸钾和水混合后的反应为(未配平):I2+KClO3+H2O→KH(IO3)2+KCl+Cl2↑。该反应是氧化还原反应,由于在氧化还原反应中电子转移数目相等,而有两种还原产物生成。它们相对量的多少不同,配平时的系数就不同。因此在该方程式配平时,系数有多组。
(2)晶体①为KH(IO3)2,把晶体从溶液中过滤出来,洗涤干净,然后用水溶解得到KH(IO3)2的溶液,要把酸式盐变为正盐KIO3,就要加碱来调节溶液的pH值。为了不引入新的杂质,所以要用KOH。该反应的方程式为KH(IO3)2+KOH=2KIO3+H2O
(3)反应I2+KClO3+H2O→KH(IO3)2+KCl+Cl2↑产生的氯气跟KOH反应生成KClO, KClO能将KIO3氧化成KIO4从而不能得到碘酸钾。导致操作失败,前功尽弃。
(4)检验加碘食盐中的碘元素,学生甲利用碘酸钾与碘化钾在酸性条件下发生反应KIO3+5KI+3H2SO4→3K2SO4+3I2+3H2O。由于I2容易溶解在四氯化碳而不容易溶解在水中,水与四氯化碳互不相溶,CCl4的密度比水大,I2溶解在CCl4中而使溶液呈紫色.所以用四氯化碳检验碘单质时,看到的明显现象有液体分层,下层液体呈现紫红色。
(5)由方程式得关系式:KIO3~3I2~6S2O32-.n(S2O32-)=2.0×10-3mol/L×0.010L=2.0×10-5mol.所以n(KIO3)= 2.0×10-5mol÷6=1/3×10-5mol.m(I)= (1/3×10-5mol)×127g/mol=127/3×10-5g=127/3×10-2mg.所以在该加碘食盐中碘的含量为m(I)÷m(总)= 127/3×10-2mg÷W×10-3kg=1270/3W mg/kg。
(6)① 该食盐中无KIO3,而实验中产生蓝色现象的可能是空气中的氧气在酸性条件下把I-氧化为I2单质。4KI+2H2SO4+O2=2K2SO4+3I2+3H2O。碘单质遇淀粉变蓝色。该反应的离子方程式为4I-+4H+ +O2=2I2+2H2O。②乙的实验中加入过量KI溶液,根据学生丙的实验结果,可知空气中的氧气也会导致产生一部分的碘单质。所以学生乙的实验结果比真实值会偏大。
