One bag, that’s it.

The importance of packing light cannot be overemphasized(过度强调). Limit yourself to 20 pounds in a carry-on bag. A 9’× 22’ ×14’ bag fits under most airplane seats. And after you enjoy that sweet mobility and freedom, you’ll never go any other way.
You’ll walk with your luggage(行李) more than you think you will. Before leaving home, give yourself a test. Pack up completely, and practice being a tourist for an hour. Fully loaded, you should enjoy window-shopping. If you can’t, go home and thin things out.
Packing light isn’t just about the trip over and back—it’s about your traveling lifestyle. Too much luggage marks you as a typical tourist. It slams the back door shut. Changing locations becomes an important operation. With only one bag, you’re mobile and in control. Take this piece of advice seriously. Pack light, and pack smart. These days, you can’t bring anything possibly dangerous—such as knives, lighters or large amounts of liquid (液体)—in your bag.
What to bring?
How do you fit a whole trip’s worth of luggage into a small backpack or suitcase(衣箱)? The answer is simple: bring very little.
Bring out everything you think you might need on the floor. Pick up one item(件) at a time and check it. Ask yourself—not “Will I use it?”, but—“Will I use it enough to feel good about carrying it all the way? The world is getting really small—you can buy Colgate toothpaste, Nivea cream and Gillette razors in almost every country.
Think about what you can do without—not what will be convenient on your trip. When in doubt, leave it out.
小题1:What do the last two paragraphs mainly talk about?
A.Packing light is a kind of travelling lifestyle.
B.Packing light can give you mobility and freedom.
C.How to find out what to take and what not to take on a trip.
D.How to find out if your luggage is too heavy.小题2:You are allowed to take _______with you when you travel according to the passage.
A.Colgate toothpaste
B.Lighters
C.Knives
D.a great deal of liquid小题3:The underlined part “thin things out” possibly means________.
A.make things thin to bring
B.make your luggage lighter
C.take out dangerous things
D.throw useless things away小题4:The author seems to believe that _________.
A.people had better bring nothing when they travel
B.people can use Nivea cream only in their home town
C.the importance of packing light is overemphasized now
D.you will be easily seen as a tourist with a lot of luggage小题5:What is the best title for the passage?
A.Dying for a trip
B.Things to take on a trip
C.Pack light and pack right
D.Travelling: a typical way of life
 、OH
、OH 和SO
和SO
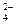 .请设计实验,探究该吸收液中可能存在的其他阴离子(不考虑空气的CO2的影响).
.请设计实验,探究该吸收液中可能存在的其他阴离子(不考虑空气的CO2的影响). ;假设3:SO32-、ClO
;假设3:SO32-、ClO 都存在。
都存在。 L-1H2SO4、1moL
L-1H2SO4、1moL L-1NaOH、0.01mol
L-1NaOH、0.01mol L-1KMnO4、淀粉-KI
L-1KMnO4、淀粉-KI 溶液、紫色石蕊试液.
溶液、紫色石蕊试液. 步骤1:取少量吸收液于试管中,滴加3 moL
步骤1:取少量吸收液于试管中,滴加3 moL L-1 H2SO4至溶液呈酸性,然后将所得溶液分置于A、B试管中.
L-1 H2SO4至溶液呈酸性,然后将所得溶液分置于A、B试管中. L-1KMnO4
L-1KMnO4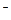 +4H+ ="== " Cl2 + Mn2+ + 2H2O
+4H+ ="== " Cl2 + Mn2+ + 2H2O )=14.2g/71g·mol
)=14.2g/71g·mol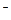 =0.2(mol)
=0.2(mol)