卤素单质的性质活泼,卤素的化合物应用广泛,运用化学反应原理研究卤族元素的有关性质具有重要意义。
(1)下列关于氯水的叙述正确的是_______(填写序号)。
A.氯水中存在两种电离平衡
B.向氯水中通入SO2,其漂白性增强
C.向氯水中通入氯气,c( H+)/c(ClO-)减小
D.加水稀释氯水,溶液中的所有离子浓度均减小
E.加水稀释氯水,水的电离平衡向正反应方向移动
F.向氯水中加少量固体NaOH,可能有c(Na+)=c(Cl- )+c(ClO-)
(2)工业上通过氯碱工业生产氯气,其反应的离子方程式为______。
(3)常温下,已知25℃时有关弱酸的电离平衡常数:

写出84消毒液(主要成分为NaClO)露置在空气中发生反应的有关化学方程式________。若将84消毒液与洁厕剂(含有浓盐酸)混合使用可能会导致中毒,请用离子方程式解释有关原因___________。
(4)碘钨灯具有比白炽灯寿命长且环保节能的;特点。一定温度下,灯泡内封存的少量碘与使用过程中沉积在管壁上的钨可以发生反应: 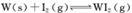 。为模拟上述反应,准确称取0. 508g碘、0.736g金属钨置于50. 0mL的密闭容器中,加热使其反应。如图是 WI2(g)的物质的量随时间变化关系图象,其中曲线I(0~t2时间段)的反应温度为T1,曲线II(从t2开始)的反应温度为T2,且T2>T1。则
。为模拟上述反应,准确称取0. 508g碘、0.736g金属钨置于50. 0mL的密闭容器中,加热使其反应。如图是 WI2(g)的物质的量随时间变化关系图象,其中曲线I(0~t2时间段)的反应温度为T1,曲线II(从t2开始)的反应温度为T2,且T2>T1。则
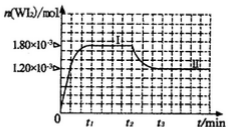
①该反应的△H_______0(填“>。、=或“<”)
②从反应开始到t1时间内的平均反应速率v(I2)=_________。
③下列说法中不正确的是_________(填序号),
A.利用该反应原理可以提纯钨
B.该反应的平衡常数表达式是K=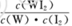
C.灯丝附近温度越高,灯丝附近区域WI2越易变为W而重新沉积到灯丝上
(5)25℃时,向5mL含有KCI和KI浓度均为0.1mol/L的混合液中,滴加6mL0.1mol/L的AgNO3溶液,先生成的沉淀是_________,溶液中离子浓度由大到小的顺序是_______ [不考虑H+和OH-。25℃时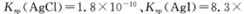
 ]。
]。
(1)A E F
(2)2Cl-+2H2O 2OH-+Cl2↑+H2↑
2OH-+Cl2↑+H2↑
(3)NaClO+CO2+ H2O=HClO+NaHCO3 ; 2HClO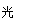 2HCl+O2↑。ClO-+Cl-+2H+= Cl2↑+H2O。
2HCl+O2↑。ClO-+Cl-+2H+= Cl2↑+H2O。
(4)① < ② 0.036/t1mol/(L·min) ③ B
(5)AgI c(K+)> c(NO3-)> c(Cl-)> c(Ag+)> c(I-).
题目分析:(1)在氯水中存在两种弱电解质,H2O、HClO。它们都存在着电离平衡H2O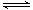 H++OH-;HClO
H++OH-;HClO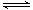 H++ClO-。正确。B. 向氯水中通入SO2,发生反应:Cl2+SO2+2H2O=H2SO4+2HCl,产生的H2SO4和HCl都没有漂白性。因此漂白作用减弱。C.向氯水中通入氯气,平衡:Cl2+H2O
H++ClO-。正确。B. 向氯水中通入SO2,发生反应:Cl2+SO2+2H2O=H2SO4+2HCl,产生的H2SO4和HCl都没有漂白性。因此漂白作用减弱。C.向氯水中通入氯气,平衡:Cl2+H2O 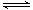 H++Cl-+HClO正向移动,c(H+) 增大,HClO
H++Cl-+HClO正向移动,c(H+) 增大,HClO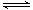 H++ClO-逆向移动,c(ClO-) 减小,c( H+)/c(ClO-)增大。错误。D.加水稀释氯水,溶液中的H+、ClO-、Cl-离子浓度减小,但由于c(H+)·c(OH-)=Kw.所以c(OH-)增大。错误。E.加水稀释氯水,由于Cl2+H2O
H++ClO-逆向移动,c(ClO-) 减小,c( H+)/c(ClO-)增大。错误。D.加水稀释氯水,溶液中的H+、ClO-、Cl-离子浓度减小,但由于c(H+)·c(OH-)=Kw.所以c(OH-)增大。错误。E.加水稀释氯水,由于Cl2+H2O 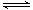 H++Cl-+HClO正向移动,酸电离产生的c(H+) 减小。对水的抑制作用减弱,所以水的电离平衡向正反应方向移动。正确。F.向氯水中加少量固体NaOH,发生反应Cl2+NaOH= NaCl+NaClO。若恰好完全反应则有c(Na+)=c(Cl- )+c(ClO-)。正确。所以正确选项为A、E、F。(2)氯碱工业生产氯气,其反应的离子方程式为2Cl-+2H2O
H++Cl-+HClO正向移动,酸电离产生的c(H+) 减小。对水的抑制作用减弱,所以水的电离平衡向正反应方向移动。正确。F.向氯水中加少量固体NaOH,发生反应Cl2+NaOH= NaCl+NaClO。若恰好完全反应则有c(Na+)=c(Cl- )+c(ClO-)。正确。所以正确选项为A、E、F。(2)氯碱工业生产氯气,其反应的离子方程式为2Cl-+2H2O 2OH-+ Cl2↑+H2↑.(3)由电离平衡常数H2CO3>HClO>HCO3-,可知:H2CO3>HClO,因此84消毒液露置在空气中发生反应的有关化学方程式NaClO+CO2+ H2O=HClO+NaHCO3。2HClO
2OH-+ Cl2↑+H2↑.(3)由电离平衡常数H2CO3>HClO>HCO3-,可知:H2CO3>HClO,因此84消毒液露置在空气中发生反应的有关化学方程式NaClO+CO2+ H2O=HClO+NaHCO3。2HClO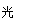 2HCl+O2↑若将84消毒液与洁厕剂(含有浓盐酸)混合使用可能会发生反应:ClO-+Cl-+2H+= Cl2↑+H2O产生有毒的气体Cl2导致中毒。(4)①因为升高温度,WI2的平衡含量降低,说明升高温度,化学平衡向逆反应方向移动。根据平衡移动原理,升高温度,化学平衡向吸热反应方向移动,逆反应方向为吸热反应,所以正反应为放热反应,因此ΔH <0. ②从反应开始到t1时间内的平均反应速率v(I2) =v(WI2)= Δc/Δt=1.80×10-3mol/0.05L÷t1min="0.036/t1mol/(L·min)" .③A. 使用不纯的的W与I2发生反应制取气体WI2,当反应达到平衡后升高温度,平衡逆向移动,就能产生纯净的单质W。因此可利用该反应原理可以提纯钨。正确。B. 由于W是固体,浓度不会改变,所以该反应的平衡常数表达式是
2HCl+O2↑若将84消毒液与洁厕剂(含有浓盐酸)混合使用可能会发生反应:ClO-+Cl-+2H+= Cl2↑+H2O产生有毒的气体Cl2导致中毒。(4)①因为升高温度,WI2的平衡含量降低,说明升高温度,化学平衡向逆反应方向移动。根据平衡移动原理,升高温度,化学平衡向吸热反应方向移动,逆反应方向为吸热反应,所以正反应为放热反应,因此ΔH <0. ②从反应开始到t1时间内的平均反应速率v(I2) =v(WI2)= Δc/Δt=1.80×10-3mol/0.05L÷t1min="0.036/t1mol/(L·min)" .③A. 使用不纯的的W与I2发生反应制取气体WI2,当反应达到平衡后升高温度,平衡逆向移动,就能产生纯净的单质W。因此可利用该反应原理可以提纯钨。正确。B. 由于W是固体,浓度不会改变,所以该反应的平衡常数表达式是 。错误。C.灯丝附近温度越高,结构平衡移动原理可知:灯丝附近区域WI2就会逆向移动,产生更多的W而重新沉积到灯丝上。正确。(5)由于Ksp(AgI)< Ksp(AgI).所以首先产生的沉淀为AgI。在混合前n(K+)=(5mL+5mL)×10-3L/mL×0.1mol/L=0.001mol;n(Ag+)=n(NO3-)=0.006L×0.1mol/L=0.0006mol;n(Cl-)=n(I-)=0.005L×0.1mol/L=0.0005mol;在混合溶液中K+、NO3-不消耗,发生反应:Ag++I-=AgI↓;Ag++Cl-=AgCl↓.根据消耗的微粒的物质的量,可确定溶液中离子的浓度大小关系为:c(K+)> c(NO3-)> c(Cl-)> c(Ag+)> c(I-).
。错误。C.灯丝附近温度越高,结构平衡移动原理可知:灯丝附近区域WI2就会逆向移动,产生更多的W而重新沉积到灯丝上。正确。(5)由于Ksp(AgI)< Ksp(AgI).所以首先产生的沉淀为AgI。在混合前n(K+)=(5mL+5mL)×10-3L/mL×0.1mol/L=0.001mol;n(Ag+)=n(NO3-)=0.006L×0.1mol/L=0.0006mol;n(Cl-)=n(I-)=0.005L×0.1mol/L=0.0005mol;在混合溶液中K+、NO3-不消耗,发生反应:Ag++I-=AgI↓;Ag++Cl-=AgCl↓.根据消耗的微粒的物质的量,可确定溶液中离子的浓度大小关系为:c(K+)> c(NO3-)> c(Cl-)> c(Ag+)> c(I-).
